Bifunctional catalyst, preparation method thereof and metal-air battery
A bifunctional catalyst and metal material technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of high cost, high noble metal content, poor catalytic cycle stability, etc., and achieve simple methods, reduce the use of precious metals, and adapt Effects on large-scale production and promotion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
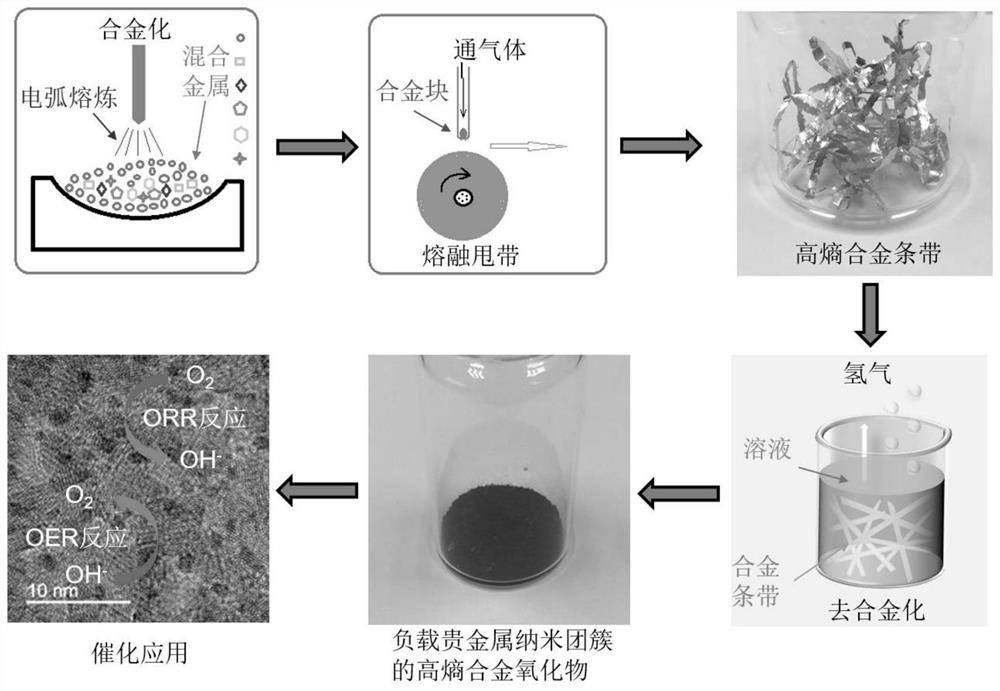
[0029] refer to figure 1 , the first embodiment of the present invention provides a method for preparing a bifunctional catalyst, comprising the following steps:
[0030] S1: Melting a variety of metal materials in proportion to obtain a homogeneous alloy body, the various metal materials include aluminum, cobalt, iron, X1, X2, wherein the X1 is selected from any two of chromium, nickel, and molybdenum Or three, the X2 is selected from one or more of platinum, palladium, gold, silver, copper; preferably, the atomic number ratio of the aluminum, cobalt, iron, X1, X2 is
[0031] 95.8~95.9:1:1:2:0.1~0.2; optimally, the X1 is chromium and molybdenum;
[0032] S2: rapidly cooling the alloy body;
[0033] S3: placing the rapidly cooled alloy body in an alkaline solution for dealloying treatment to obtain the bifunctional catalyst.
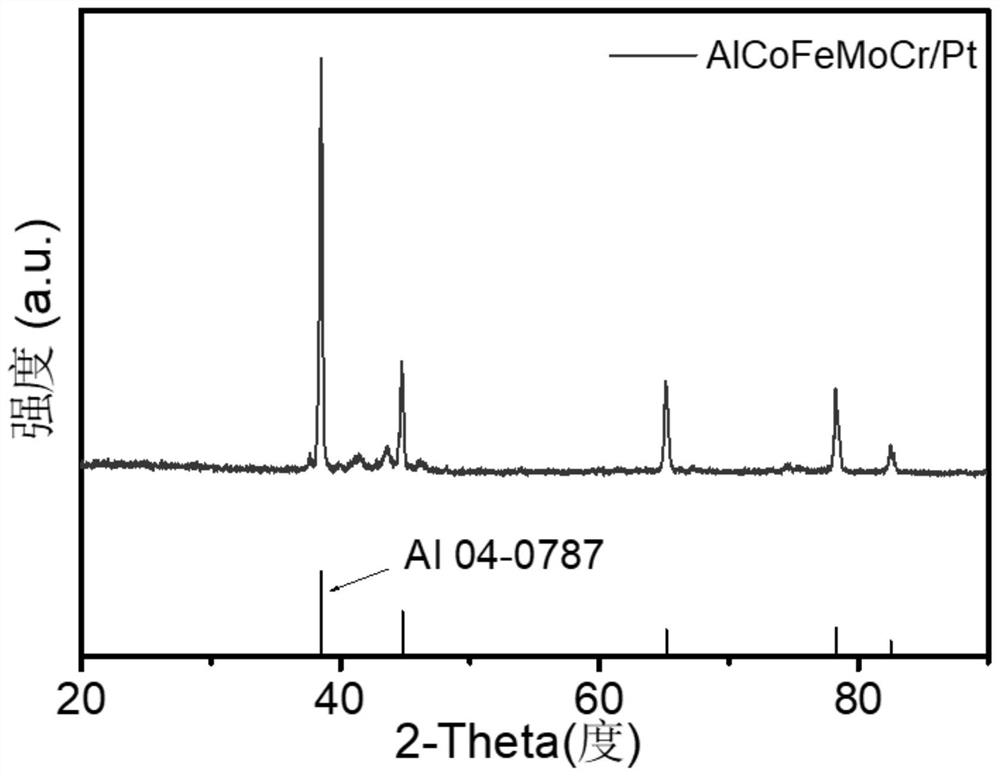
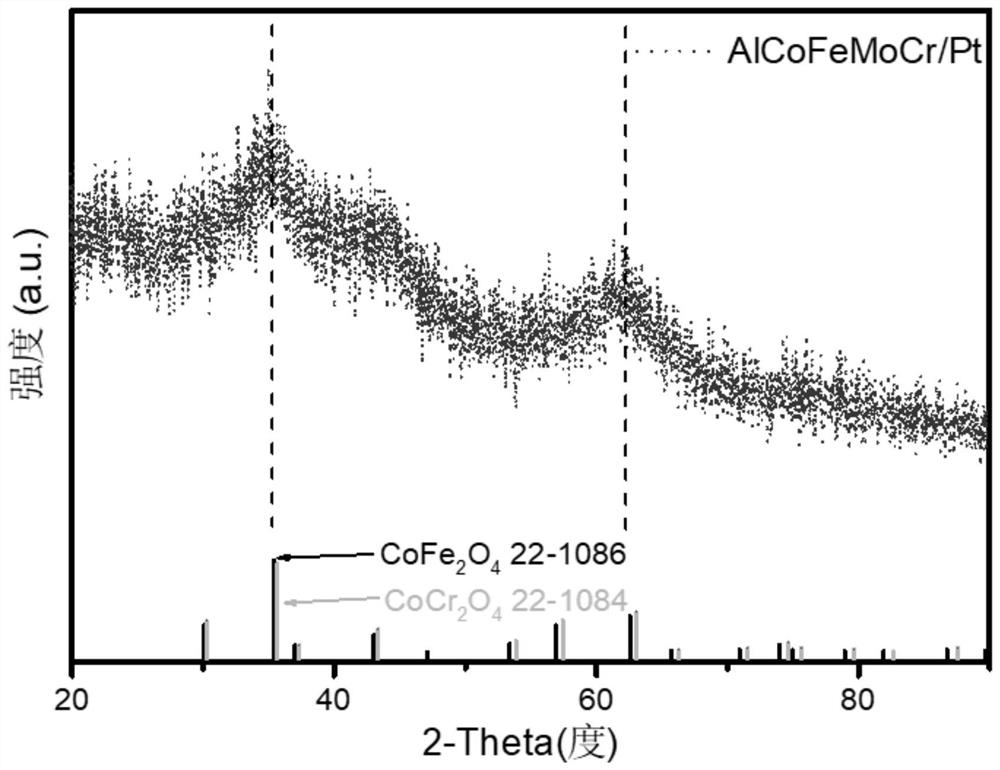
[0034] In this embodiment, in step S1, the various metal materials to be smelted are aluminum, cobalt, iron, molybdenum, chromium, and platinum metal...
Embodiment 2
[0051] This embodiment is substantially similar to Embodiment 1, and the similarities will not be repeated here. The main difference between the two lies in that the types and proportions of the various metal materials to be smelted are different. In this embodiment, the various metal materials for smelting are aluminum, cobalt, iron, molybdenum, chromium, platinum with an atomic number ratio of 95.8:1:1:1:1:0.04:0.04:0.04:0.04:0.04 , palladium, gold, silver, copper metal particles.
Embodiment 3
[0053] This embodiment is substantially similar to Embodiment 1, and the similarities will not be repeated here. The main difference between the two lies in that the types and proportions of the various metal materials to be smelted are different. In this embodiment, the various metal materials to be smelted are aluminum, cobalt, iron, nickel, chromium, and platinum metal particles with an atomic number ratio of 95.9:1:1:1:1:0.1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com