Method for preparing cell substrate, method for preparing kit and joint detection method
A kit and cell technology, applied in the field of immunoassay, can solve the problems of high specificity, long time-consuming separation and culture method, unfavorable promotion, etc., achieve the effect of shortening the time and avoiding the interference of non-specific fluorescence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
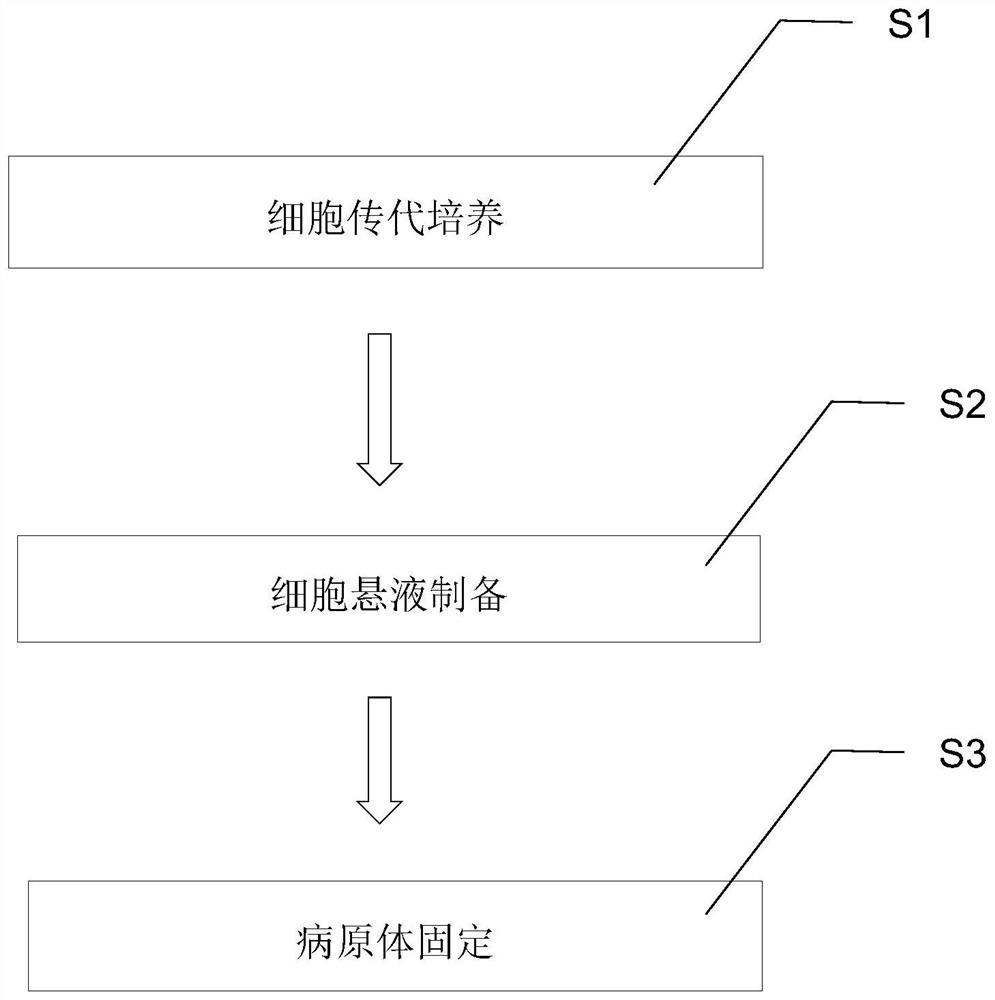
[0094] The preparation of a cell suspension infected with non-bacteria involves the following steps:
[0095] In step S21, at least two kinds of passaged cells (for example, the passaged cells obtained in step S12) among various pathogens are digested and separated with trypsin at a concentration ranging from 0.1% to 0.5%, preferably at a concentration of 0.25%. The at least two kinds of passaged cells are detached from the bottom of the culture vessel (for example, the bottom of the culture bottle), and stop digestion and separation when they appear in a three-dimensional shape, and suck out the trypsin;
[0096] In step S22, the digested and separated at least two types of passaged cells are respectively placed in 5% CO with a humidity ≥ 90%, preferably a humidity of 96% and a temperature of 37°C. 2 Adsorption in the incubator for 1h to 3h, preferably 2h;
[0097] In step S23, supplement the adsorbed at least two kinds of passaged cells with fetal bovine serum medium in a c...
Embodiment 1
[0119] 1. The method for preparing the cell substrate for joint detection of respiratory infection comprises the following steps:
[0120] (1) Subculture of cells:
[0121] Method 1 Pathogen-infected cell culture: Add 10% fetal bovine serum MDEM medium to Hep-2, MDCK, MK2, and A549 cells to be fixed, and then place them at a temperature of 37°C, a humidity of 40%, and CO 2 Concentration of 5% CO 2 Cultivate in the incubator for 48 hours to recover; then digest and separate with 0.25% trypsin, aspirate the trypsin and count 6 million / T75, and place it again in 5% CO with a temperature of 37°C and a humidity of 90%. 2 Cultured in an incubator for 24 hours for subculture.
[0122] Method 2: Cultivate Legionella pneumophila LP cells: After the LP special medium powder is dissolved, carry out autoclaving at 121°C for 15 minutes, pour it into a plate after cooling, and make about 15ml of medium in each plate. After the medium solidifies, Inoculate 50uL of LP bacteria and place in...
Embodiment 2
[0143] Common pathogens of enteric infection include rotavirus RV, adenovirus ADV, astrovirus Astrovirus, and enterovirus 71 EV71.
[0144] 1. The preparation method of the cell substrate for joint detection of intestinal infection comprises the following steps:
[0145] (1) Subculture of cells:
[0146] Method 1 Pathogen-infected cell culture: Add 10% fetal bovine serum MDEM medium to the MA-104, ADV, MK2, and Caco-2 cells to be fixed, and then place them in a 5% medium with a temperature of 37°C and a humidity of 98%. CO 2 Cultivate in the incubator for 48 hours to recover; then digest and separate with 0.25% trypsin, aspirate the trypsin and count 6 million / T75, and place it again in 5% CO with a temperature of 37°C and a humidity of 98%. 2 Cultured in an incubator for 24 hours for subculture.
[0147] (2) Pathogen-infected cells:
[0148] To inoculate and infect the cells by selecting half of the virus seed amount of TCID50, and digest and separate the cells before the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com