Method for detecting cyclosporine pharmaceutical preparation and auxiliary materials thereof
A pharmaceutical preparation and detection ring technology, applied in the field of analysis and detection in medicinal chemistry, can solve the problems of unfavorable drug quality research and reverse research, no detectable method, etc., and achieve the effect of simple operation, high efficiency and good sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
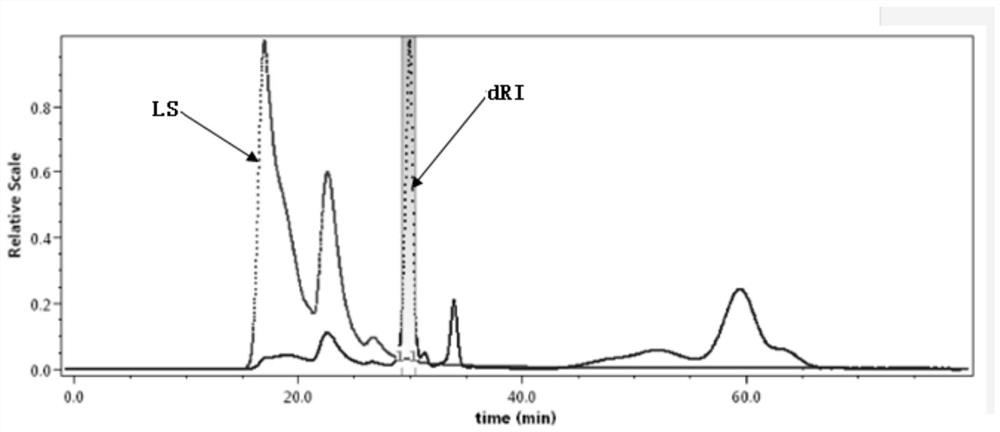
[0082] After freeze-drying 10mL cyclosporine eye drops, in 2mL0.05M NaNO 3Shake and dissolve in the solution; prepare a solution containing OP-40 at a concentration of 2.5mg / mL, povidone at a concentration of 15mg / mL, and polyoxyethylene hydrogenated castor oil at a concentration of 50mg / mL, as the test solution, let it stand for 30min, and use Filter through a 0.22 micron microporous membrane and pour into the sample bottle.
[0083] Take 100 μL of the filtered test solution obtained by the above method. Detection was performed by gel chromatography exclusion method. Record the chromatogram as figure 1 As shown, the analysis and separation of the excipients in the cyclosporine eye drops sample was completed.
[0084] Detection conditions:
[0085] Chromatographic column: Waters chromatographic column Ultrahydrogel 250 and Ultrahydrogel 500 connected in series;
[0086] Mobile phase: 0.1M NaNO 3 Aqueous solution (pH9, pH regulator: Na 2 HPO 4 );
[0087] Column temper...
Embodiment 2
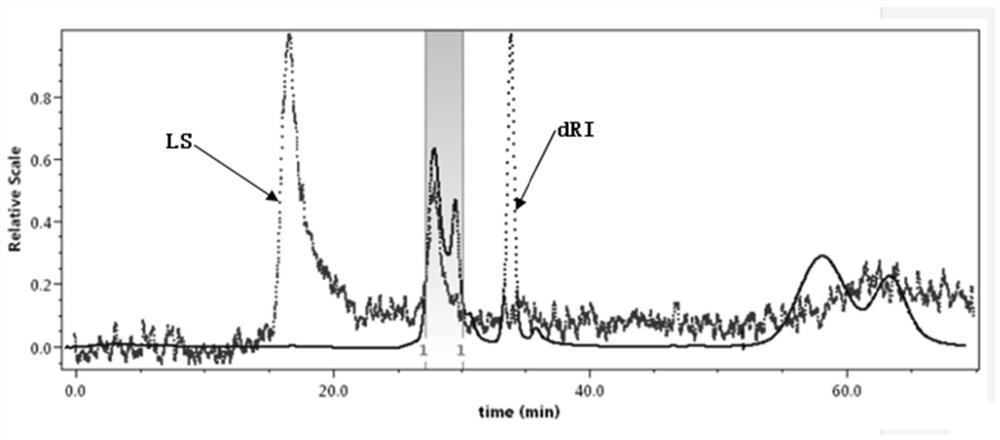
[0094] Take an appropriate amount of OP40 to a 10ml volumetric flask, and use 0.05M NaNO 3 Shake and dissolve in the solution, prepare OP-40 with a concentration of 5.0mg / mL as the test solution, let it stand for 30min, filter it with a 0.22 micron microporous membrane, and inject it into the sample bottle.
[0095] Take 100 μL of the filtered test solution obtained above, and detect it by gel chromatography exclusion method. Record the chromatogram, the result is as follows figure 2 As shown, the detection of the OP-40 sample was completed.
[0096] Detection conditions:
[0097] Chromatographic column: Waters chromatographic column Ultrahydrogel 250 and Ultrahydrogel 500 connected in series;
[0098] Mobile phase: 0.1M NaNO 3 Aqueous solution (pH9, pH regulator: Na 2 HPO 4 );
[0099] Column temperature: set the Waters GPC column oven heater to 40°C;
[0100] Flow rate: mobile phase flow rate is 0.6mL / min;
[0101] Analysis time: 80min;
[0102] Injection volume: ...
Embodiment 3
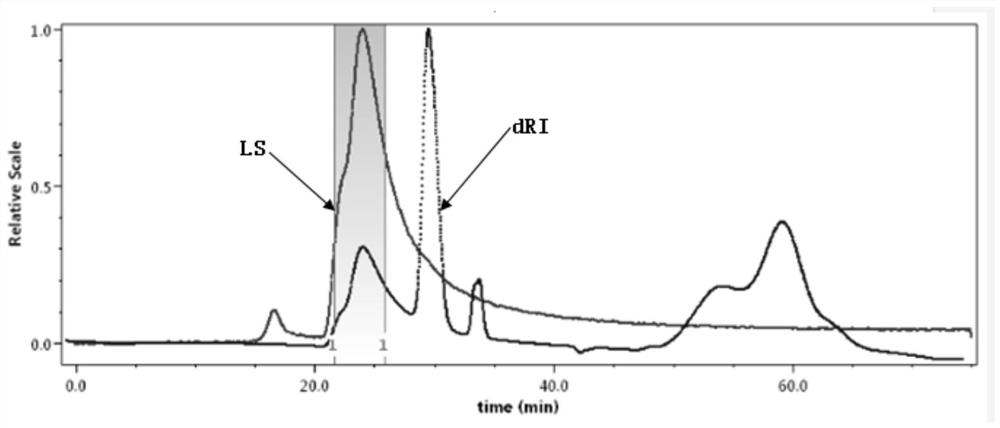
[0106] Take an appropriate amount of polyoxyethylene hydrogenated castor oil to a 10ml volumetric flask, and use 0.05M NaNO 3 Shake and dissolve in the solution, prepare polyoxyethylene hydrogenated castor oil with a concentration of 50mg / mL as the test solution, let it stand for 30min, and inject it into the sample bottle after filtering with a 0.22 micron microporous filter membrane.
[0107] Take 100 μL of the filtered test solution obtained above and use the gel chromatography exclusion method to detect with the following detection conditions. Record the chromatogram, the result is as follows image 3 shown.
[0108] Chromatographic conditions:
[0109] Chromatographic column: Waters chromatographic column Ultrahydrogel 250 and Ultrahydrogel 500 connected in series;
[0110] Mobile phase: 0.1M NaNO 3 Aqueous solution (pH9, pH regulator: Na 2 HPO 4 );
[0111] Column temperature: set the Waters GPC column oven heater to 40°C;
[0112] Flow rate: mobile phase flow ra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com