Anti-human abnormal prothrombin antibody and its application
An abnormal prothrombin and antibody technology, applied in applications, anti-enzyme immunoglobulins, instruments, etc., can solve the problem of no specific detection of human DCP antibodies, so as to avoid the interference of the source of abnormal prothrombin and reduce the difficulty , the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
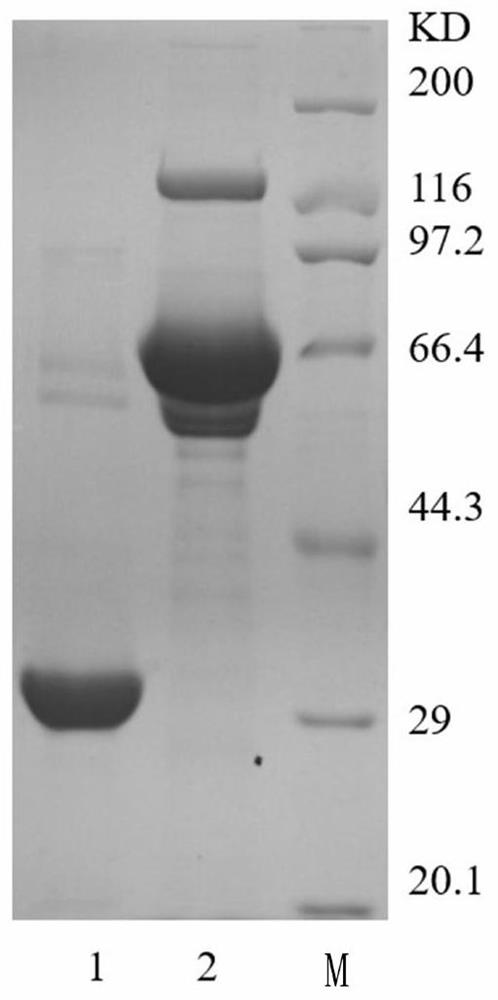
[0051] Example 1 Preparation of Abnormal Prothrombin Recombinant Antigen
[0052] According to the prothrombin sequence (Accession number: NM_000506.5) retrieved from Gene Bank, recombinant expression cloning was designed, and the full-length sequence is shown in SEQ ID NO:9. According to the differences between DCP and normal prothrombin mainly in the first 50 amino acids, and the reported antibody epitopes are generally linear epitopes, the commercial vectors pColdTF and pGEX-20T vectors were used to construct prothrombin 1-50aa respectively The expression plasmid of the E. coli expression system is used to express the target protein, because the protein cannot be modified by gamma carboxylation in E. coli, so the expression product is abnormal prothrombin, DCP corresponding 1-50aa, the specific nucleotide sequence Shown in SEQ ID NO:10.
[0053] Because the two expression vectors both use the same restriction site, the primers are the same. Primer5.0 is used to design prim...
Embodiment 2
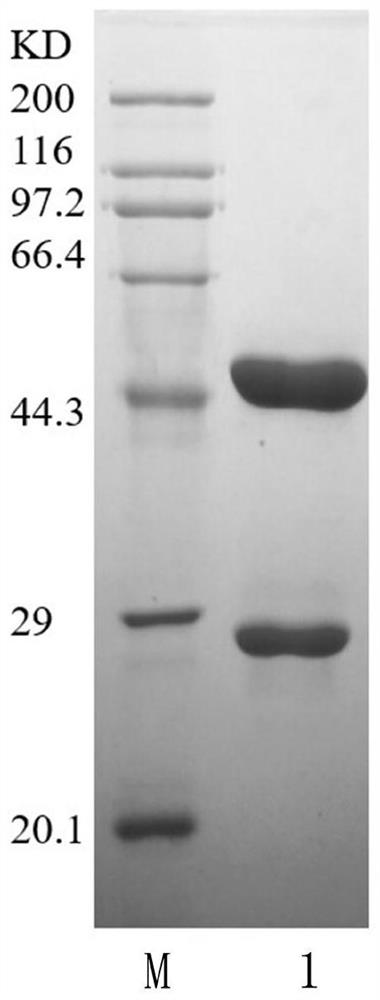
[0064] Example 2 Preparation of Abnormal Prothrombin Monoclonal Antibody
[0065] 1. Preparation of the immunogen: the immunogen is the antigen 1 prepared in Example 1. Antigen 1 was diluted to 0.4mg / mL with 10mmol / L PBS, and the corresponding antigen was mixed with Freund's adjuvant in equal volumes according to the final concentration of 200ug / ml to form a water-in-oil emulsion. Complete Freund's adjuvant was used for the initial immunization, and incomplete Freund's adjuvant was used for the booster immunization.
[0066] 2. Basic immunization: BALB / c female mice aged 6-8 weeks were selected for subcutaneous multi-point immunization. The dose of immunogen injection was 500 μL / mouse / time, the immunization interval was 2 weeks, and the complete immunization procedure was 4 injections. 2 weeks after the 3rd and 4th doses of immunization, the isolated serum was collected by orbital blood collection method for indirect ELISA to determine the immune titer. After the mouse serum ...
Embodiment 3
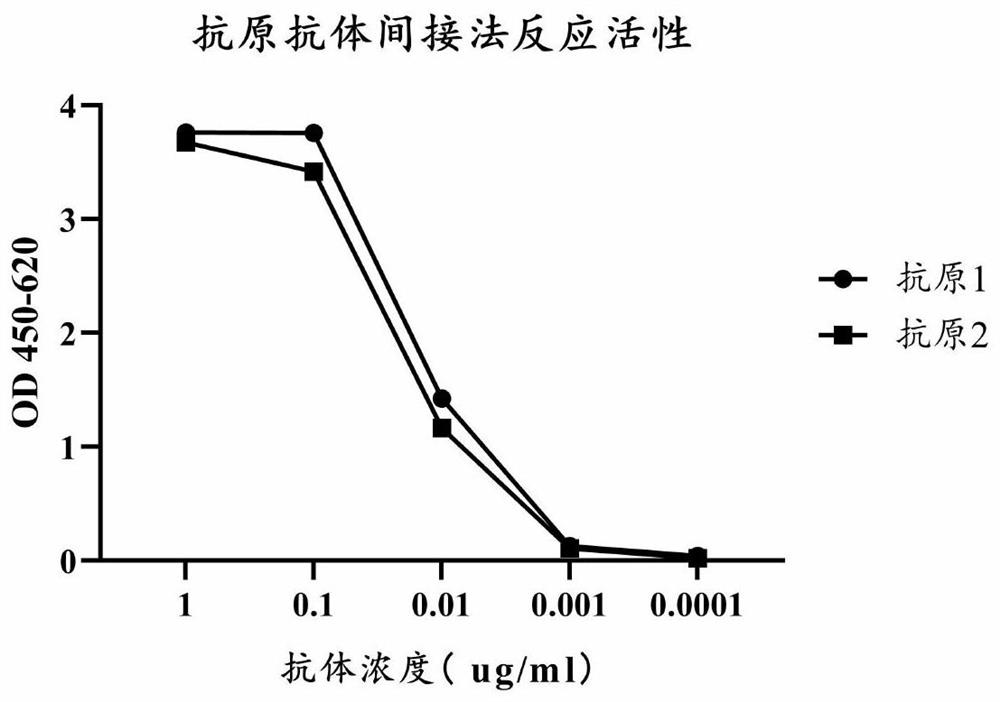
[0097] Example 3 Monoclonal Antibody Epitope Identification
[0098] 1. Identification of 15 peptides
[0099] Antigen 1 was diluted with carbonic acid buffer (20mmol / L CB, pH 9.6) and coated on a polyvinyl chloride plate at 200ng / ml. The purified antibody was mixed with the synthesized DCP1-50aa polypeptide (Shenggong Synthetic, the length of the polypeptide is 15aa, synthesized by overlapping 5 amino acids successively, as shown in the underline in the table, the specific sequence information is shown in Table 1) Proportional 100ng / ml antibody: 50ug / ml polypeptide is incubated in equal volume for 30min, which is the test sample; the control sample is 100ng Antibody / ml: PBS, incubate for 30 minutes, add the incubated test sample and control sample to the plate coated with antigen 1 and incubate for 30 minutes, wash the plate 5 times, add GAM-HRP (1 / 5000 dilution) and incubate for 30 minutes , wash the plate 5 times, add substrate and incubate for 15 min. Read the value of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com