Application of famitinib in preparation of medicine for treating tumor with c-KIT or PDGFRA mutation
A use and technology of tinib, which can be applied in the direction of antineoplastic drugs, drug combinations, pharmaceutical formulations, etc., and can solve problems such as treatment failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: Cell Proliferation Inhibition Experiment
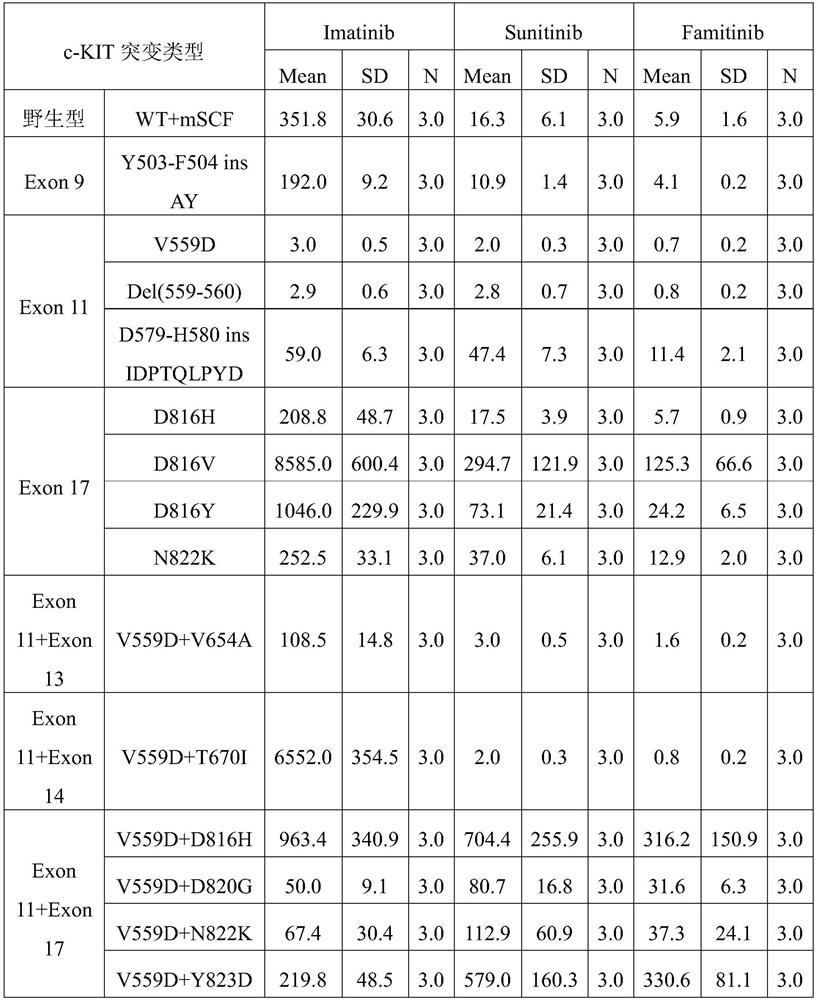
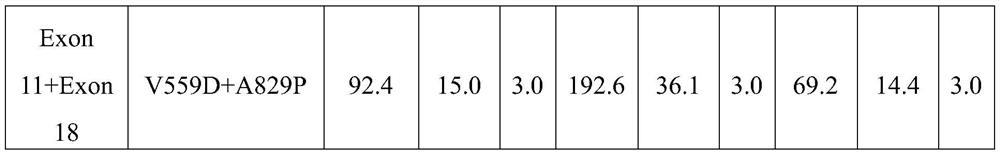
[0041] 1. Famitinib, sunitinib and imatinib inhibit the proliferation of 32D cells transfected with various c-KIT mutations
[0042] Materials and methods
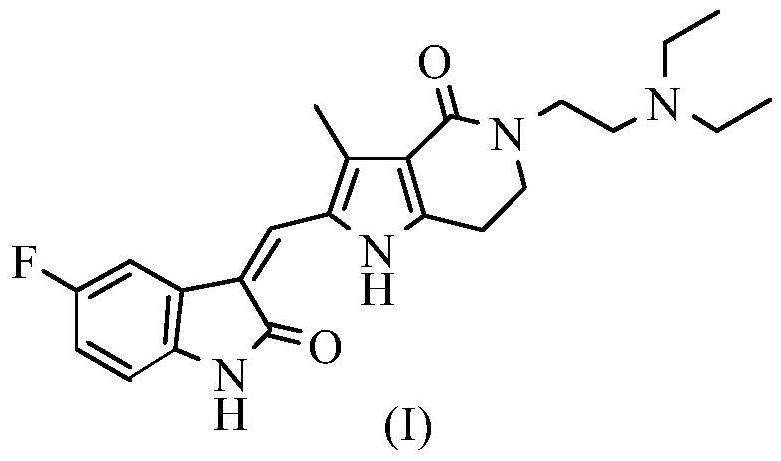
[0043] Compounds: famitinib malate, imatinib mesylate and sunitinib malate were synthesized and provided by Jiangsu Hengrui Pharmaceutical Co., Ltd. (Jiangsu Province, China).
[0044] site-directed mutagenesis
[0045] Michael H. Tomasson (Washington University School of Medicine, St. Louis, MO, USA) generously provided a murine stem cell virus-based retroviral construct carrying a mouse-human heterozygous WT KIT cDNA or the activating mutant D816V (816Asp→ Val) KIT cDNA. A heterozygous KIT allele is generated by in-frame fusion of the extracellular and transmembrane domains of murine KIT with the intracellular domain of human KIT. Studies have shown that replacement of the human extracellular and transmembrane domains of KIT with homologous murine sequen...
Embodiment 2
[0065] Example 2: Multicenter, open, single-arm phase II clinical trial dosing regimen of famitinib malate in the treatment of gastrointestinal stromal tumors
[0066] Famitinib 25 mg orally before breakfast daily. The dose can be lowered to 20mg / d according to the side effects; continuous administration for 28 days is a treatment cycle, and the drug should be taken at roughly the same time every day as much as possible. During the period, if toxic side effects occur, the administration can be suspended, and the suspension of administration time will be included in the treatment cycle. The initial dose of famitinib is 25 mg, and if the dose needs to be lowered due to toxicity, it is allowed to be lowered to 20 mg / d.
[0067] Inclusion criteria
[0068] Patients must meet all of the following inclusion criteria to be enrolled in the trial:
[0069] 1) Male or female, aged 18-75 years;
[0070] 2) Patients with metastatic recurrence / unresectable GIST diagnosed by histopathol...
Embodiment 3
[0114] Example 3: A randomized, open-label, controlled, multi-center phase III clinical study of famitinib malate versus sunitinib malate in the treatment of gastrointestinal stromal tumors that failed imatinib treatment
[0115] Dosing regimen
[0116] test group:
[0117] Famitinib: Oral administration, 25mg / time, once a day, before or after meals. It is recommended to take the drug at a fixed time every day, within 0.5 hours after a meal, and every 6 weeks is a treatment cycle. Dose adjustments and dosing delays are allowed.
[0118] Control group:
[0119] Sunitinib: Oral administration, 50mg / time, once a day, can be administered before or after meals, it is recommended to administer at a fixed time every day, taking 4 weeks and stopping for 2 weeks, every 6 weeks is a treatment cycle. Dosage adjustment and delayed administration are allowed, please refer to the drug instructions for specific administration and adjustment schemes.
[0120] Inclusion criteria
[0121...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diastolic pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com