DR8 polypeptide analogue as well as preparation method and application thereof
A technology of analogs and drugs, applied in the field of biochemistry, can solve the problems such as the need to improve the drug efficacy, the poor stability of DR8, and the low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Embodiment 1, the preparation of compound
[0082] Step 1: The synthesis of the compound adopts the Fmoc solid-phase synthesis method, and is synthesized from the carboxyl terminal to the amino terminal direction. The specific steps are as follows:
[0083] (1) Activation of MBHA resin: Weigh the resin and add an appropriate amount of DCM to swell on the shaker for 30 minutes, drain and add DMF to wash 3 times, each time for 3 minutes;
[0084] (2) Indene test: add ninhydrin: pyridine: phenol = 1:2:1 indene test reagent in the test tube, dip a little resin in it, and bathe in boiling water for 3 minutes. If the indene test result is yellow, it indicates that the resin is normal;
[0085] (3) Resin deprotection: add DMF solution containing 3% double-distilled piperidine to remove the protective group, remove the residual reagent, add DMF to wash, 3 min each time, repeat 4 times, and remove the residual reagent;
[0086] (4) Indene test: add ninhydrin: pyridine: phenol =...
Embodiment 2
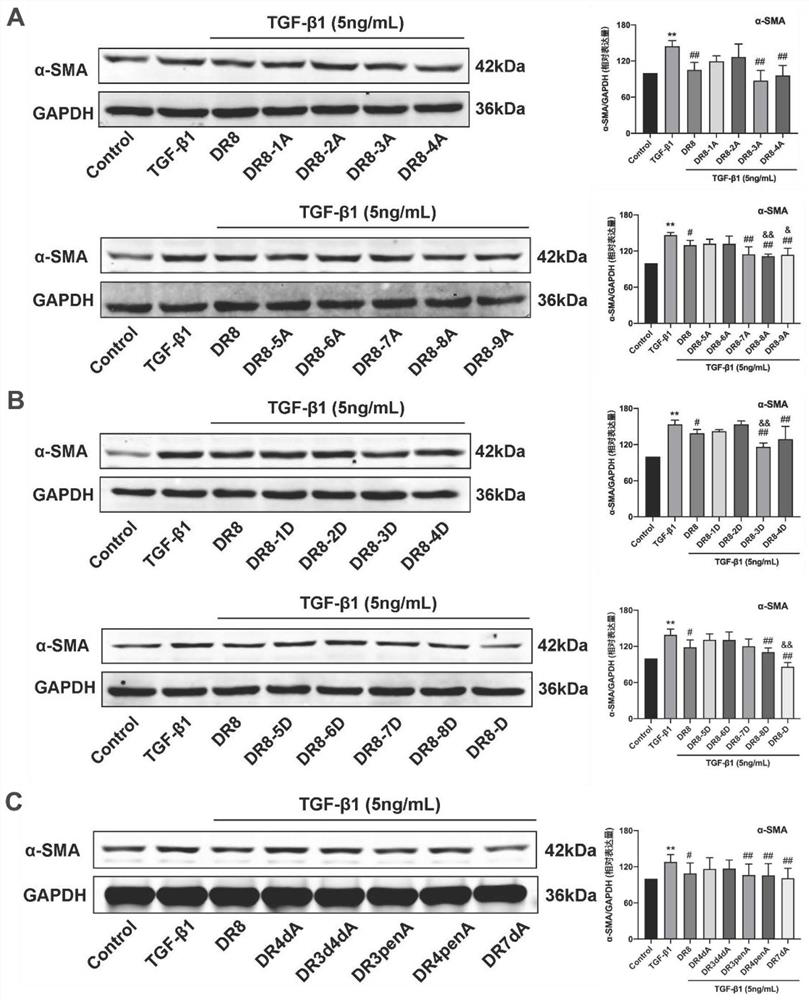
[0102] Embodiment 2, using the compound obtained in Embodiment 1 as the test substance to carry out the in vitro activity screening experiment of fibrosis
[0103] The mouse embryonic fibroblast NIH3T3 cell line was selected to study and observe the effect of the test substance on the expression of α-smooth muscle actin (α-smooth muscle actin, α-SMA) in NIH3T3 cells induced by transforming growth factor TGF-β1.
[0104] NIH3T3 cells were plated in 6-well plates with DMEM + 10% FBS + 1% double antibody medium (ThermoFisher) at 37°C, 5% CO 2 After culturing for 24 hours under the same conditions, replace it with serum-free medium for 12 hours, add 5ng / mL LTGF-β1 and 80μM test substance to act on the cells for 48 hours, extract the total protein of the cells, and detect the protein expression level of α-SMA by Western blot.
[0105] Experiments include:
[0106] Blank control group( figure 1 Marked as Control, TGF-β1 and test substance were not added in the culture medium);
...
Embodiment 3
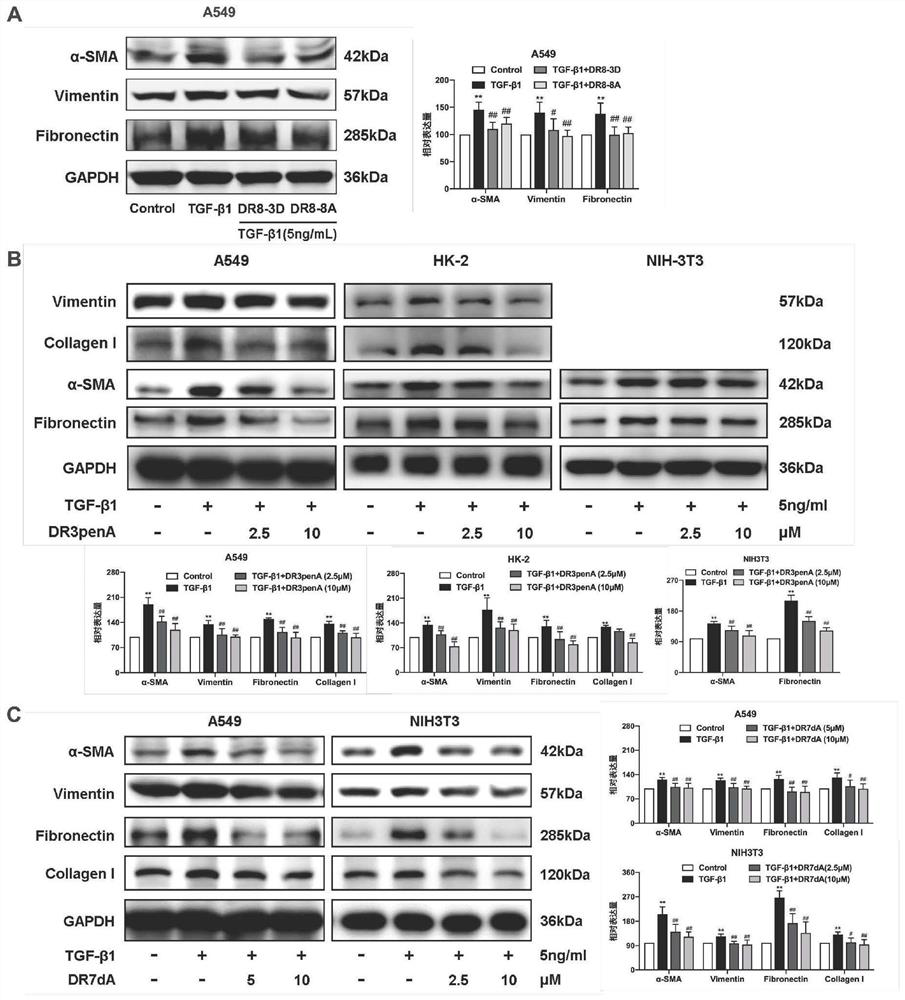
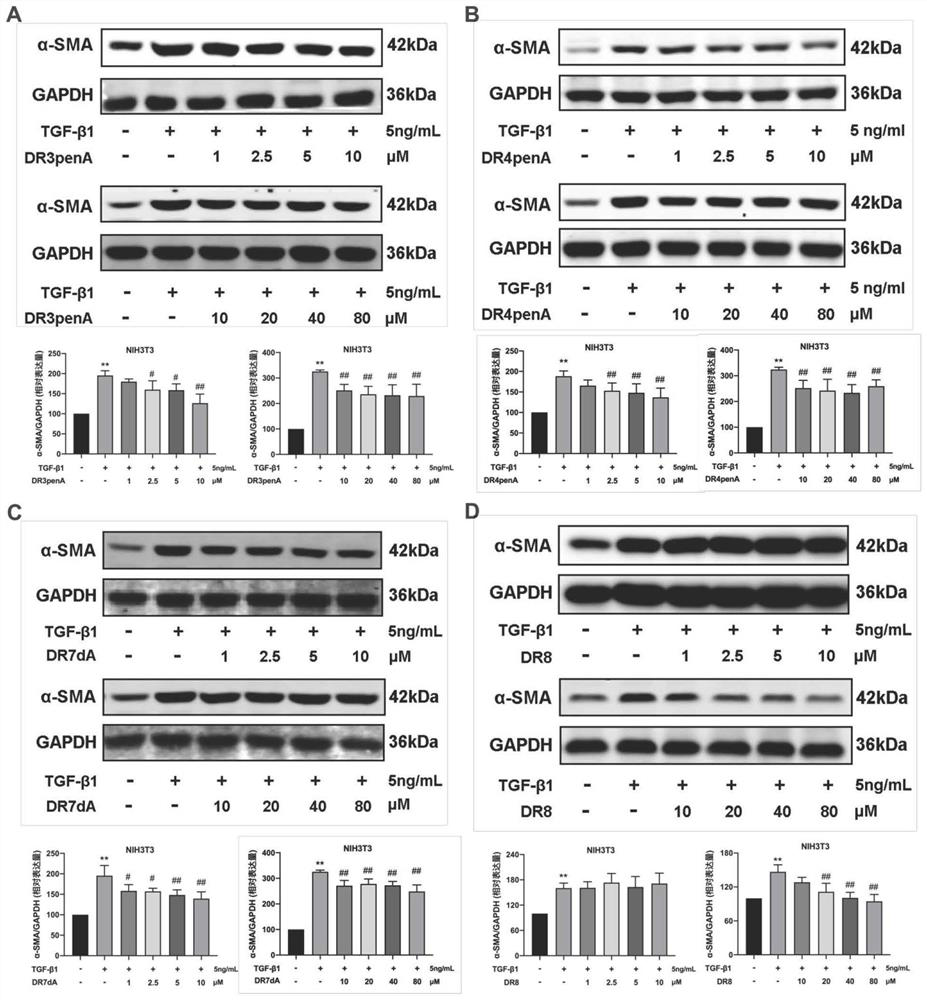
[0114] Example 3. The compounds DR8-3D, DR8-8A, DR3penA, DR4penA and DR7dA obtained in Example 1 were used as test substances to perform in vitro inhibitory effect experiments on fibrosis
[0115] Human lung adenocarcinoma cell line A549, renal tubular epithelial cell line HK-2 and embryonic fibroblast cell line NIH3T3 were selected to study and observe the effects of test substances on the expression of fibrosis-related proteins in different cells.
[0116] A549 cells, HK-2 cells and NIH3T3 cells were respectively plated in 6-well plates, and RPMI1640+10% FBS+1% double-antibody medium, DMEM / F12+10% FBS+1% double-antibody medium and DMEM+ 10% FBS+1% double antibody medium at 37°C, 5% CO 2 Cultivate for 24 hours under the same conditions, replace with serum-free medium for 12 hours, add 5ng / mL LTGF-β1 and different concentrations of test substances to act on A549 cells or NIH3T3 cells for 48 hours, or act on HK-2 cells for 24 hours, then extract the total cell protein, and dete...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com