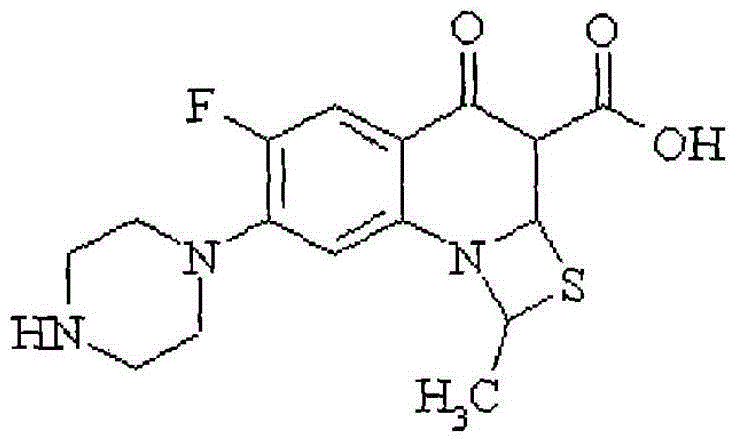
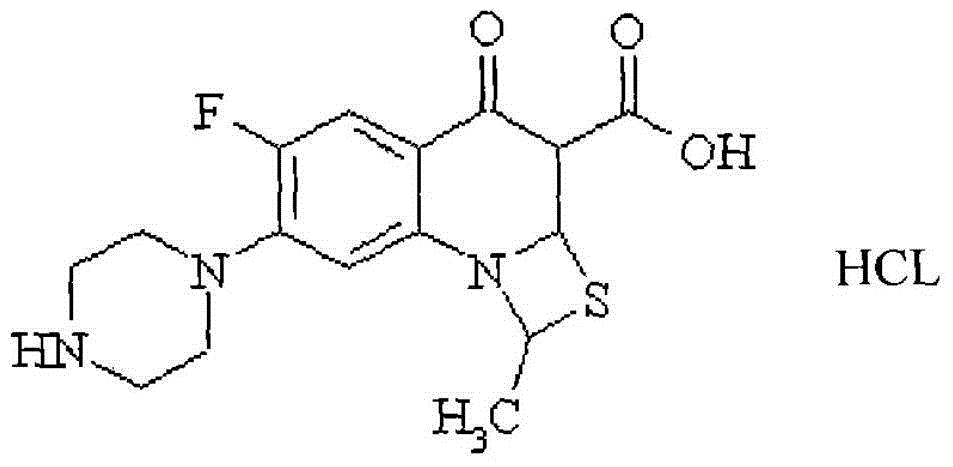
A kind of Ulifloxacin hydrochloride eye drops and preparation method thereof
A technology of ulifloxacin hydrochloride and eye drops, which is applied in directions such as pharmaceutical formulations, medical preparations containing active ingredients, and drug delivery, can solve problems such as side effects and drug resistance, achieve low toxic and side effects, a convenient and simple production process, Low cytotoxic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Ulifloxacin hydrochloride (prepared from sterile powder raw materials):
[0061] Add 5L of water and 1.5L of acetone into the reaction tank, add 1kg of Ulifloxacin powder at 10-15°C, add 1.5L of 2mol / L hydrochloric acid dropwise under stirring, stir for 1.5 hours, add 0.05kg of activated carbon, stir for 20 minutes, filter Decarburization; the filtrate is filtered through a 0.22um microporous membrane to a crystallization tank in a sterile room. Under stirring, 35 L of acetone was added dropwise until solid precipitated, and the addition was completed in 4 hours. Insulate at 0-5°C and stir for 5 hours, filter, wash twice with 6L of acetone, and dry under vacuum at 35°C to obtain 0.84 kg of raw material of sterile powder of ulifloxacin hydrochloride.
Embodiment 2
[0063] Ulifloxacin hydrochloride eye drops contain 3 mg of ulifloxacin hydrochloride, 40 mg of polyvinylpyrrolidone, and 0.3 mg of ethylparaben per milliliter.
[0064] prescription:
[0065] Ulifloxacin Hydrochloride 30g
[0066] Polyvinylpyrrolidone (Povidone PVP) 400g
[0067] Ethylparaben 3g
[0068] Appropriate amount of glycerin
[0069] Appropriate amount of acetic acid-sodium acetate buffer (acetate buffer, pH5.5)
[0070] Add water for injection to 10L.
[0071] Preparation Process:
[0072] Take 30g of ulifloxacin hydrochloride and dissolve it in 2L of water for injection; take 400g of polyvinylpyrrolidone, 3g of ethylparaben, and 300ml of glycerin and dissolve it in 5L of water for injection, heat to dissolve; combine the two liquids, filter, and add acetic acid- Sodium acetate buffer solution, add water for injection to 10L, check the content of the semi-finished product to pass the test, adjust the pH value to 5.5, sterilize by steam at 100°C for 30 minutes,...
Embodiment 3
[0075] Ulifloxacin hydrochloride eye drops, the finished product contains 1mg of ulifloxacin hydrochloride, 40mg of polyvinylpyrrolidone, and 0.4mg of ethylparaben per milliliter.
[0076] prescription:
[0077] Ulifloxacin Hydrochloride 5g
[0078] Polyvinylpyrrolidone 200g
[0079] Ethylparaben 2g
[0080] Appropriate amount of glycerin
[0081] Appropriate amount of acetate buffer
[0082] Appropriate amount of 5% sodium hydroxide solution
[0083] Add water for injection to 5L.
[0084] Preparation Process:
[0085] Dissolve 5g of Ulifloxacin Hydrochloride in 1L of Water for Injection; Dissolve 200g of Polyvinylpyrrolidone, 1.5g of Ethylparaben, and 150ml of Glycerin in 3L of Water for Injection, heat to dissolve; combine the two liquids, filter, and add vinegar from the filter Salt buffer solution, add water for injection to about 5L, adjust the pH value to 6.5±0.5 with 5% sodium hydroxide solution, check the content of the semi-finished product to pass the test, s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com