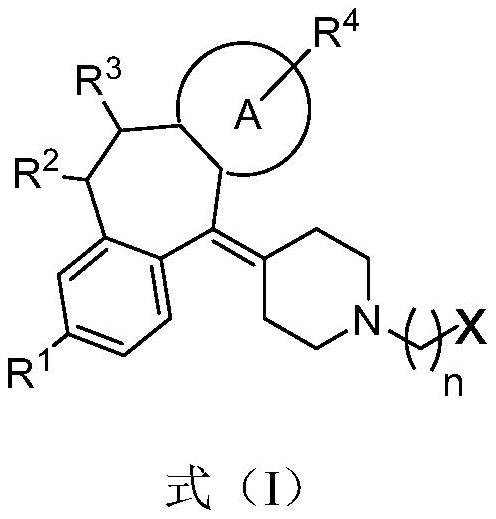
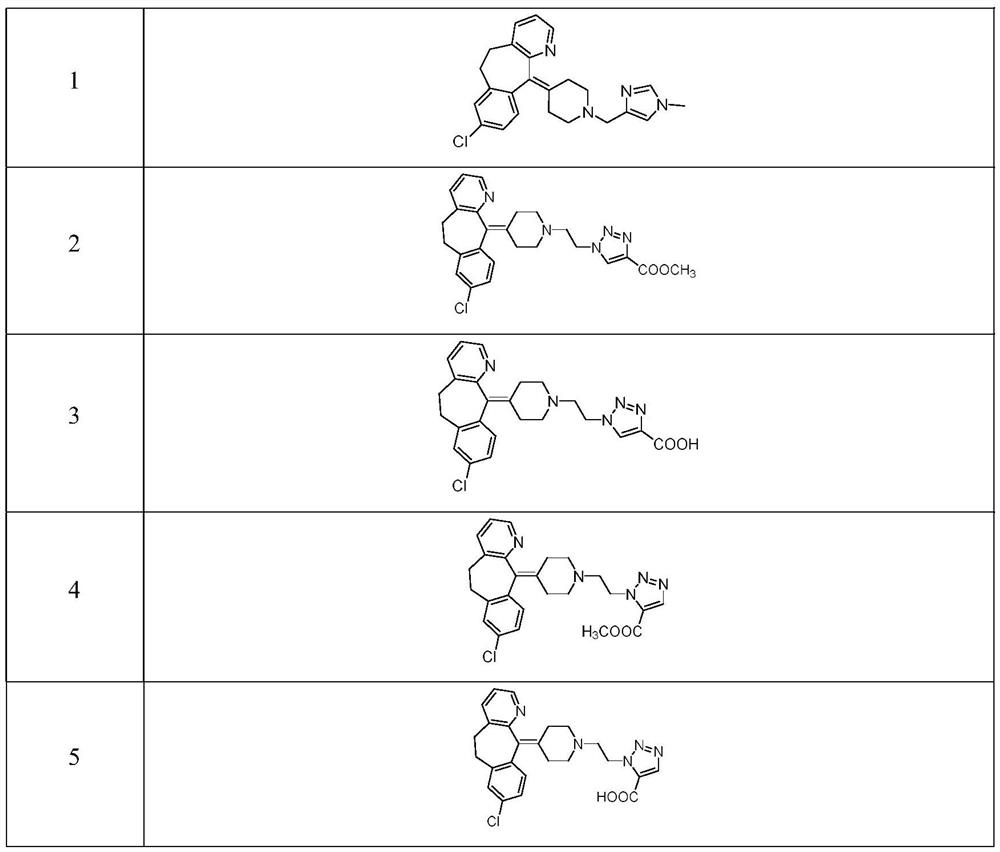
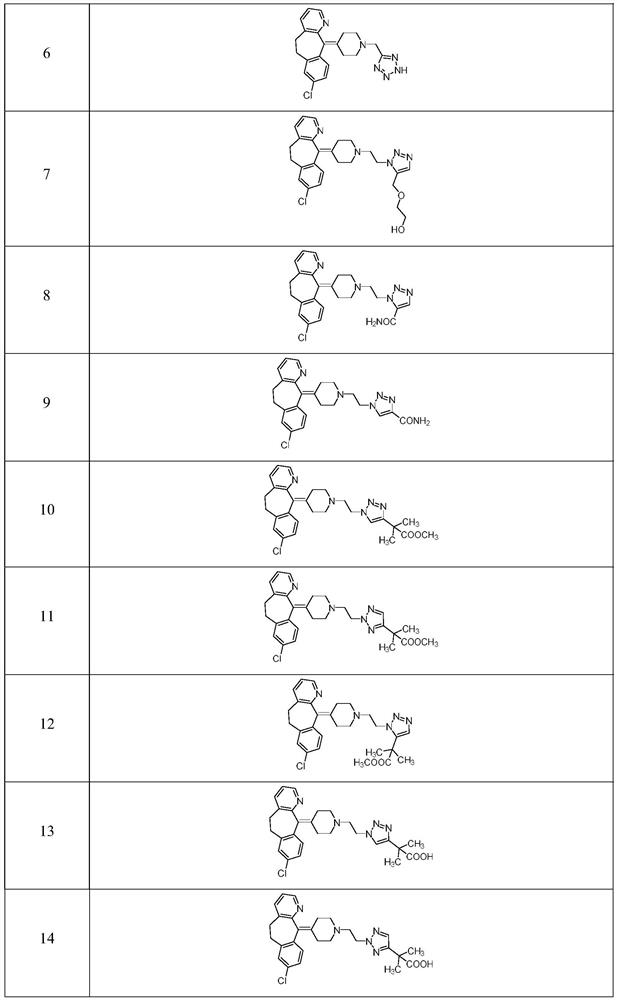
Triazole compound as well as preparation method and medical application thereof
A technology for compounds and medicinal salts, applied in the field of medicinal chemistry, can solve the problems of undisclosed compound 1 implementation method, activity data, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] 8-chloro-11- (1 - (5-methyl-1H-imidazole-4-yl) methyl) piperidine-4-subunit) -6,11-dihydro-5H-benzo [5, 6] Huan Geng [1,22-B] pyridine synthesis
[0062] Compoundllarrene (310 mg, 1 mmol), 5-methyl-1H-imidazole-4-formaldehyde (330 mg, 3 mmol) is dissolved in 1.2-dichloroethane (50 mL), and the mixture is stirred at room temperature for 10 min, then add Sodium triacetoxyborohydride (626 mg, 3 mmol), acetic acid (3 droplets). The reaction to 25 ° C was stirred overnight. LC (DCM: MeOH = 10: 1) The detection of the feedstock reaction is complete. Concentration, mixed, column layers were analyzed. Compound (190 mg, 47% yield), white solid. 1 H NMR (400MHz, CDCL 3 : ΔPPM 8.36 (DD, J 1 = 1.2Hz, J 2 = 4.8Hz, 1H), 7.74 (S, 1H), 7.47 (DD, J 1 = 0.8Hz, J 2 = 7.8 Hz, 1H), 7.47 (D, J = 2.0 Hz, 1H), 7.10-7.14 (m, 2H), 7.04 (D, J = 8.0 Hz, 1H), 4.09 (S, 2H), 3.31-3.39 (M, 2H), 3.06-3.20 (m, 4H), 2.61-2.89 (m, 6H), 2.28 (s, 3h).
Embodiment 2
[0064] Step 1 2- (4- (8-chloro-5,6-dihydro-11H-benzo [5,6] cyclohepta [1,2-B] pyridine-11-subunit) piperidine-1-yl ) Synthesis of ethane-1-alcohol (2)
[0065]
[0066] The raw material 1 (400 mg, 1.29 mmol) and N, N-diisopropylethylamine (417 mg, 3.23 mmol) were dissolved in DCM (7 mL). After stirring at room temperature for 30 min, 2-bromoethanol (404 mg, 3.23 mmol) was added. The reaction was stirred at room temperature for 22 h. TLC (V Acetone: V Dichloromethane: V Triethylamine = 1: 2: 0.1) The detection of the feedstock 1 reaction is complete. The reaction was stopped, and the carnovic solvent was stopped, and the column chromatography (V dichloromethane: v-triethylamine = 2: 1: 0.05) was isolated, and the white solid was 412 mg, the yield was 90.0%. 1 H-NMR (300MHz, CDCL 3 δ (PPM): 8.42 (D, J = 4.2 Hz, 1H, ARH), 7.46 (D, J = 7.5 Hz, 1H, ARH), 7.19-7.12 (M, 4H, ARH), 3.78-3.75 (M , 2h, ch 2 OH), 3.45-3.34 (m, 2h, Ar CH 2 ), 2.97-2.57 (M, 12H, Ar CH 2 , N (CH) 2 ) 3 , C ...
Embodiment 3
[0081] 1- (2- (4- (8-chloro-5,6-dihydro-11H-benzo [5,6] cyclohepta [1,2-b] pyridine-11-subunit) piperidine-1- Synthesis of Base) Ethyl) -1H-1,2,3-triazole-5-formate
[0082] Referring to the synthesis method of Example 2, the column chromatography (eluent: V dichloromethane: V methanol = 200: 1 ~ 50: 1) is separated, a light brown solid 210 mg compound, yield: 43.1%, MP186.2 ~ 188.1 ° C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com