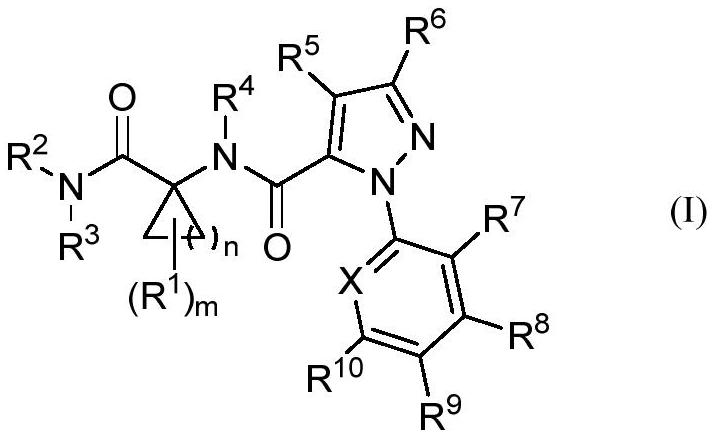
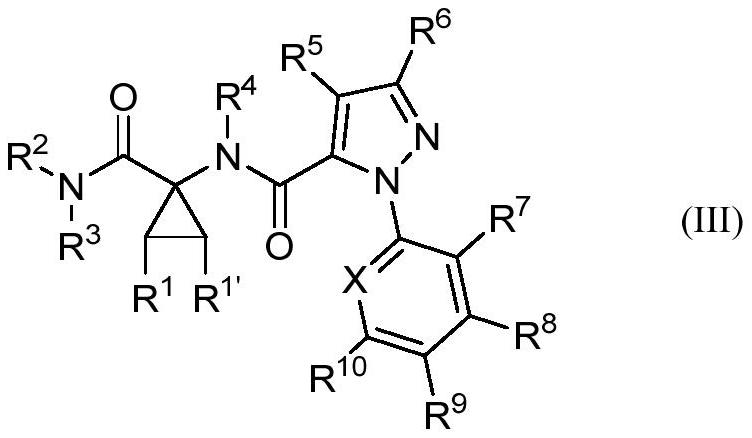
Cyclic amino acid-containing diamide compound
A compound, cycloalkyl technology, applied in the field of insecticide active agent and pest control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0139] The preparation method of the compound of the present invention
[0140] The diamide compounds containing cyclic amino acids of the present invention can be prepared by the following method, but the conditions of the method, such as reactants, solvents, bases, the amount of compounds used, reaction temperature, reaction time required, etc. are not limited to the following Explanation. The compounds of the present invention can also be conveniently prepared by combining various synthetic methods described in the specification or known in the art, and such combinations can be easily performed by those skilled in the art to which the present invention belongs.
[0141] The present invention synthesizes the diamide compounds containing cyclic amino acids represented by the general formulas (I)-(V), the preparation method is simple and easy, the operation is simple, the product is easy to purify, the cost is low, and the stability is improved. The diamide compound containin...
Embodiment 1
[0143] Embodiment 1: the preparation of 3-bromo-1-(3-chloropyridin-2-yl)-N-(1-(phenylcarbamoyl) cyclohexyl)-1H-pyrazole-5-carboxamide (compound I-1)
[0144] 1.1 Preparation of tert-butyl (1-(phenylcarbamoyl) cyclohexyl) carbamate
[0145]
[0146] 2.43g (10mmol) of 1-(tert-butoxycarbonyl)amino)cyclohexane-1-carboxylic acid, 100mL of dichloromethane, 0.91g (9.5mmol) of aniline, 3.03g (30mmol) of triethyl Amine and 5.72 g (11 mmol) of benzotriazol-1-yl-oxytripyrrolidinylphosphonium hexafluorophosphate were sequentially added into a 250 mL three-necked flask. After reacting at room temperature for 4 h, 100 mL of water was added. The organic phase was separated off and the aqueous phase was extracted twice with 50 mL of dichloromethane. The organic phases were combined, dried over anhydrous sodium sulfate, and the solvent was removed by rotary evaporation. The crude product was purified by column chromatography to obtain 2.22 g of white solid with a yield of 73.5%.
[014...
Embodiment 2
[0158] Embodiment 2: Preparation of 3-bromo-1-(3-chloropyridin-2-yl)-N-(1-(phenylcarbamoyl)cyclopentyl)-1H-pyrazole-5-carboxamide ( Compound I-3)
[0159] 2.1 Preparation of tert-butyl (1-(phenylcarbamoyl) cyclopentyl) carbamate
[0160]
[0161] The synthesis of the target compound is similar to 1.1, except that the starting material is 1-(tert-butoxycarbonyl)amino)cyclopentane-1-carboxylic acid instead of 1-(tert-butoxycarbonyl)amino)cyclohexane- 1-Carboxylic acid. The product is a white solid with a yield of 70.1%.
[0162]2.2 Preparation of 1-amino-N-phenylcyclopentane-1-carboxamide
[0163]
[0164] The synthesis of the target compound is similar to 1.2, except that the raw material is tert-butyl (1-(phenylcarbamoyl) cyclopentyl) carbamate instead of tert-butyl (1-(phenylcarbamoyl) Cyclohexyl) carbamate. The product is a yellow oily liquid with a yield of 98.3%. The next reaction was carried out directly without further treatment.
[0165] 2.3 Preparation of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com