Intrinsic luminous halide scintillation crystal and preparation method and application thereof
A technology of scintillation crystals and halides, which is applied in chemical instruments and methods, luminescent materials, crystal growth, etc., can solve the problems of reducing material energy resolution, scintillation performance degradation, instability, etc., and achieve high scintillation luminescence efficiency and high energy Effects of Resolution, High Lattice Integrity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
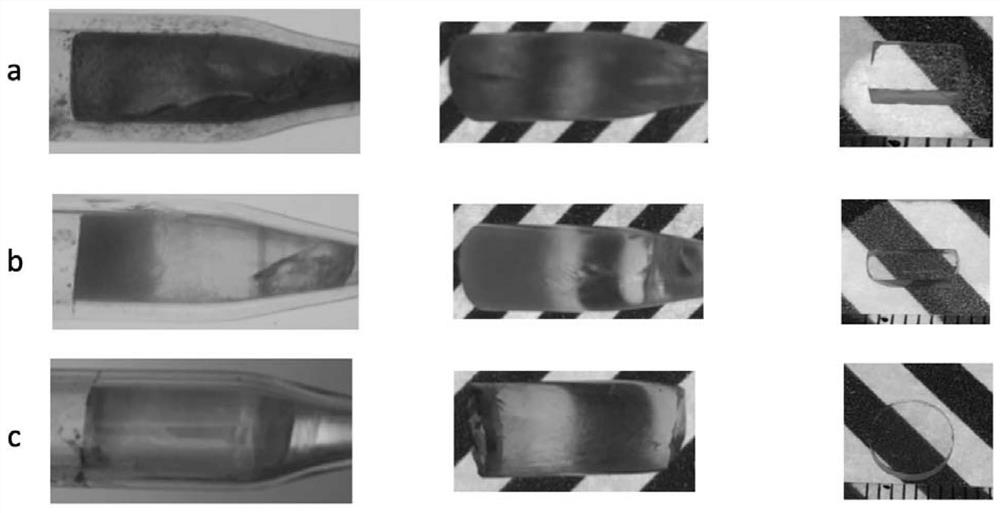
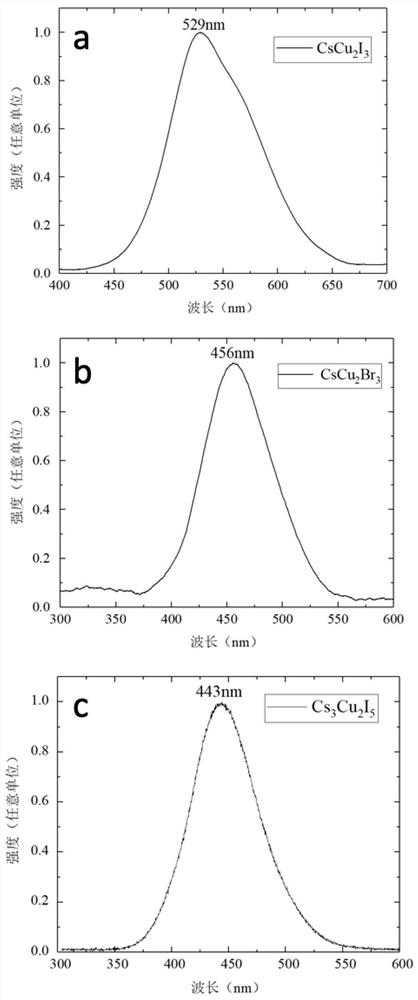
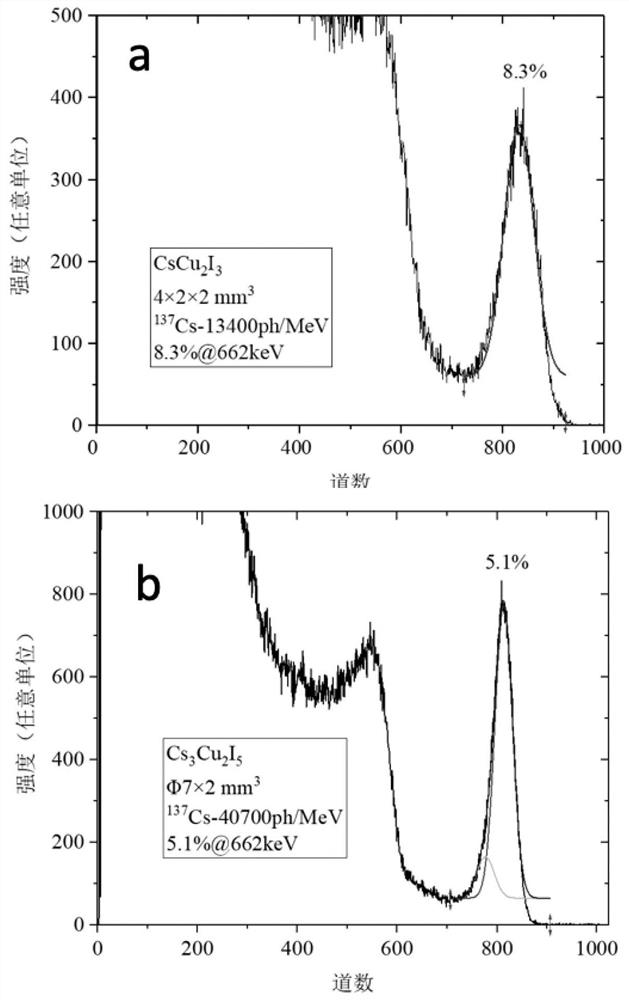
[0041] The intrinsic halide scintillation crystal proposed in this embodiment 1 has a chemical formula of CsCu 2 I 3 , namely (A 1-x A' x )(B 1- y B' y ) 2 (X 1-z X' z ) 3 Is general formula; A=Cs; B=Cu; X=I; x=y=z=0.
[0042] The above-mentioned rare earth halide mixed scintillation crystal is prepared by a crucible descent method, which includes the following steps:
[0043] a) The intrinsic halide scintillator composition chemical formula CsCu prepared on demand 2 I 3 Weigh each raw material. For specific operations, follow the CsCu 2 I 3 Weighing the high-purity raw materials CsI and CuI with a purity of 99.99% in molar ratio;
[0044] b) In an inert gas environment, place each raw material in a quartz crucible with a capillary bottom; then evacuate the inside of the crucible and seal it by welding. In this embodiment, the inert gas environment is a glove box filled with argon or nitrogen;
[0045] c) Place the welded quartz crucible vertically in the midd...
Embodiment 2
[0049] The intrinsic halide scintillation crystal proposed in Example 2 has a chemical formula of Cs 3 Cu 2 I 5 , namely (A 1-x A' x ) 3 (B 1- y B' y ) 2 (X 1-z X' z ) 5 Is general formula; A=Cs; B=Cu; X=I; x=y=z=0.
[0050] The above-mentioned rare earth halide mixed scintillation crystal is prepared by a crucible descent method, which includes the following steps:
[0051] a) Intrinsic halide scintillator composition chemical formula Cs prepared on demand 3 Cu 2 I 5 Weigh each raw material. For specific operations, follow the CsCu 2 I 3 Weighing the high-purity raw materials CsI and CuI with a purity of 99.99% in molar ratio;
[0052] b) In an inert gas environment, place each raw material in a quartz crucible with a capillary bottom; then evacuate the inside of the crucible and seal it by welding. In this embodiment, the inert gas environment is a glove box filled with argon or nitrogen;
[0053] c) Place the welded quartz crucible vertically in the midd...
Embodiment 3
[0057] The intrinsic halide scintillation crystal proposed in this example has the chemical formula of CsCu 2 Br 3 , namely (A 1-x A' x )(B 1- y B' y ) 2 (X 1-z X' z ) 3 It is a general formula; A=Cs; B=Cu; X=Br; x=y=z=0.
[0058] The above-mentioned rare earth halide mixed scintillation crystal is prepared by a crucible descent method, which includes the following steps:
[0059] a) The intrinsic halide scintillator composition chemical formula CsCu prepared on demand 2 Br 3 Weigh each raw material. For specific operations, follow the CsCu 2 Br 3 Weighing the high-purity raw materials CsBr and CuBr with a purity of 99.99% in molar ratio;
[0060] b) In an inert gas environment, place each raw material in a quartz crucible with a capillary bottom; then evacuate the inside of the crucible and seal it by welding. In this embodiment, the inert gas environment is a glove box filled with argon or nitrogen;
[0061]c) Place the welded quartz crucible vertically in t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com