Ionic type polyimine network material and preparation method thereof
An ionic, polyimide technology, applied in the production of bulk chemicals, can solve the problems of difficult and flexible electronic equipment, poor self-healing performance, etc., and achieve the effects of good electrical conductivity, easy preparation, and rapid self-healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
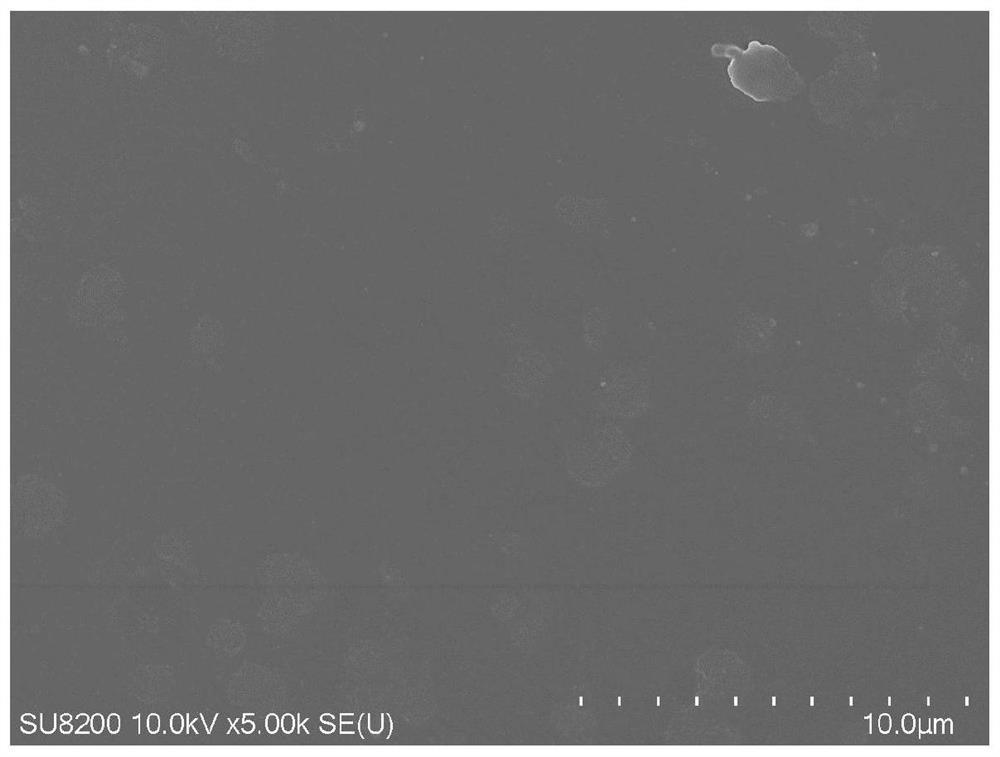
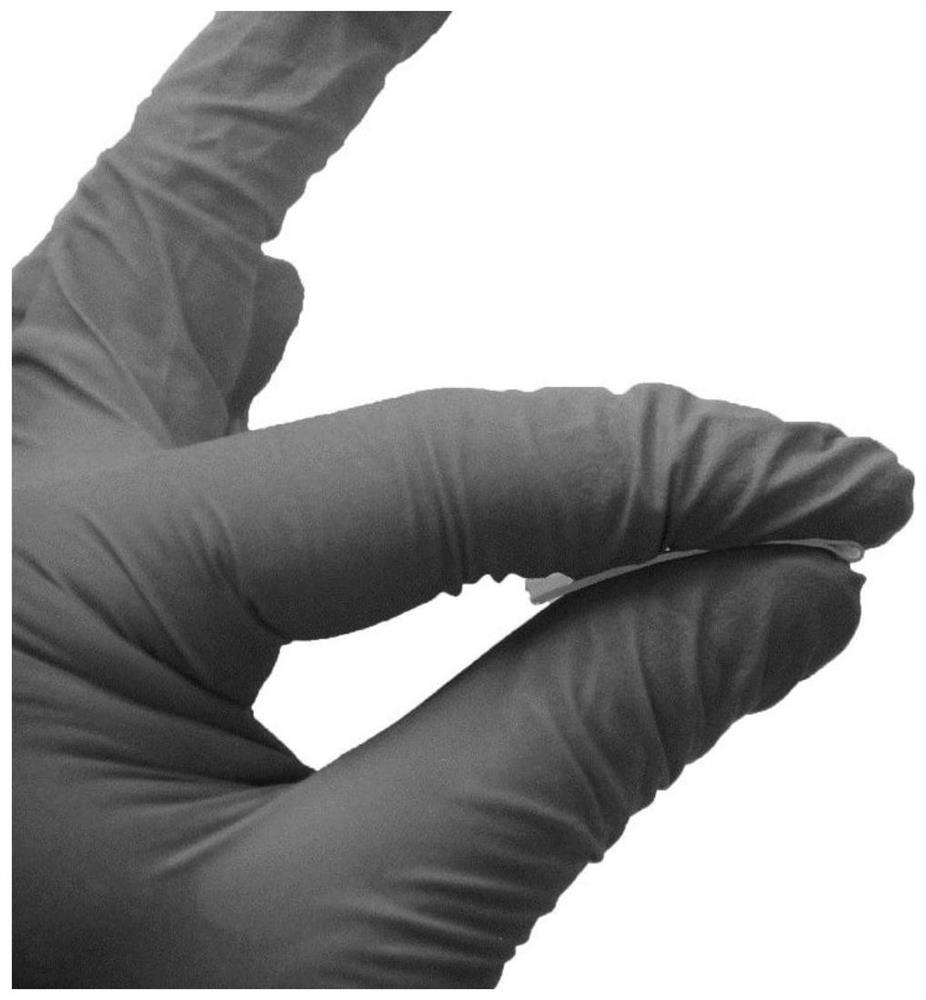
Image
Examples
Embodiment 1
[0044] A kind of preparation method of ionic polyimide network material, concrete steps are as follows:
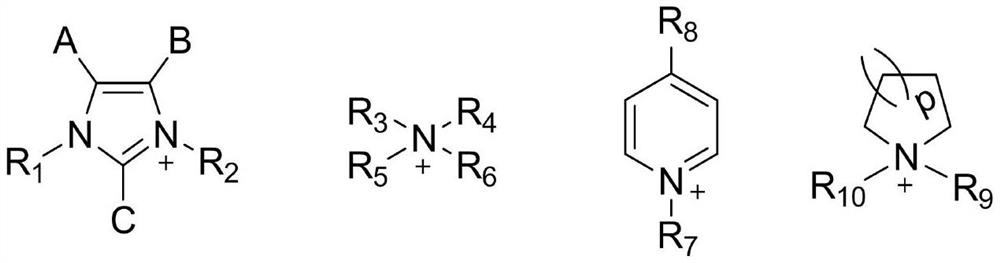
[0045] (1) Add 0.2 mmol of 1-ethyl-3-methylimidazolium lysine salt ([Emim][Lys]) (refer to the preparation method Room temperature ionic liquids from 20natural amino acids. J Am ChemSoc.2005Mar 2; 127 (8): 2398-9, the following examples can adopt similar method) to be dissolved in the middle of 2mL methanol solution, the tris (2-aminoethyl) amine of 0.4mmol is dissolved in the middle of the ethanol solution of 4mL, then two kinds Mix the amine solutions, and ultrasonicate at 60°C for 2 minutes under the ultrasonic power of 2000W to obtain a uniform, clear and transparent mixed amine solution;
[0046] (2) Another 0.8 mmol of terephthalaldehyde was dissolved in a mixed solution of 0.3 mL of dichloromethane and 1.8 mL of ethanol, and ultrasonicated at 45°C for 15 min under an ultrasonic power of 2000 W to obtain a clear light yellow aldehyde solution;
[0047] (3) Mix the a...
Embodiment 2
[0051] A preparation method of ionic polyimide network material is basically the same as that of Example 1, the difference is that:
[0052] In step (1), 1-ethyl-3-methylimidazolium 2,3-diaminopropionate is used as the amino acid derivative ionic liquid.
[0053] The ionic polyimide network material obtained in this embodiment is measured by a conductivity meter, and the obtained conductivity is 48.8mS cm -1 .
[0054]
Embodiment 3
[0056] A preparation method of ionic polyimide network material is basically the same as that of Example 1, the difference is that:
[0057] In step (1), 1-ethyl-3-methylimidazolium 2,10-diaminodecanoate is used as the amino acid derivative ionic liquid.
[0058] The ionic polyimide network material obtained in this embodiment is measured by a conductivity meter, and the obtained conductivity is 37.9mS cm -1 .
[0059]
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com