Cell surface marker for detecting circulating tumor cells of breast cancer patient and application
A cell surface and tumor cell technology, applied in the field of cell surface markers for detection of CTCs in breast cancer patients, can solve the problems of inapplicability to tumors, lack of CTCs, and inability to capture, and achieve efficient capture of breast cancer CTCs, efficient capture, and accurate information. and strong support
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
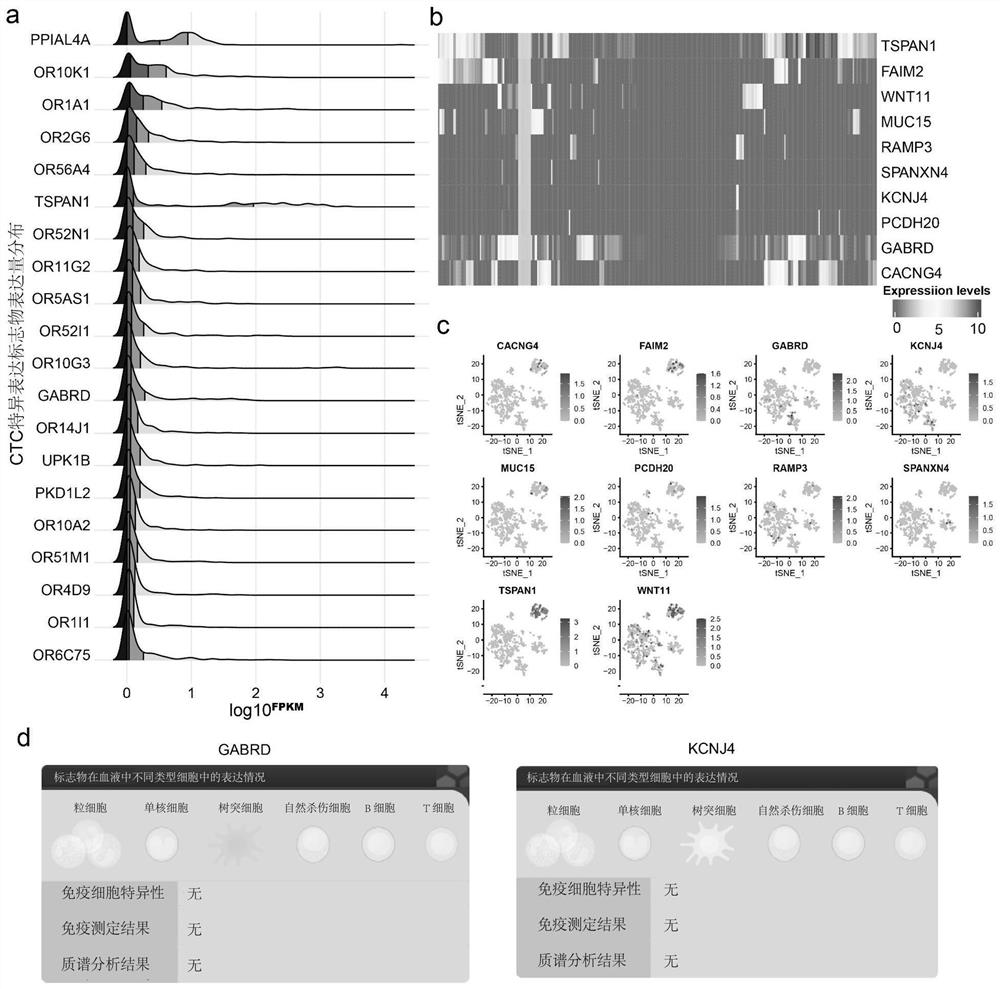
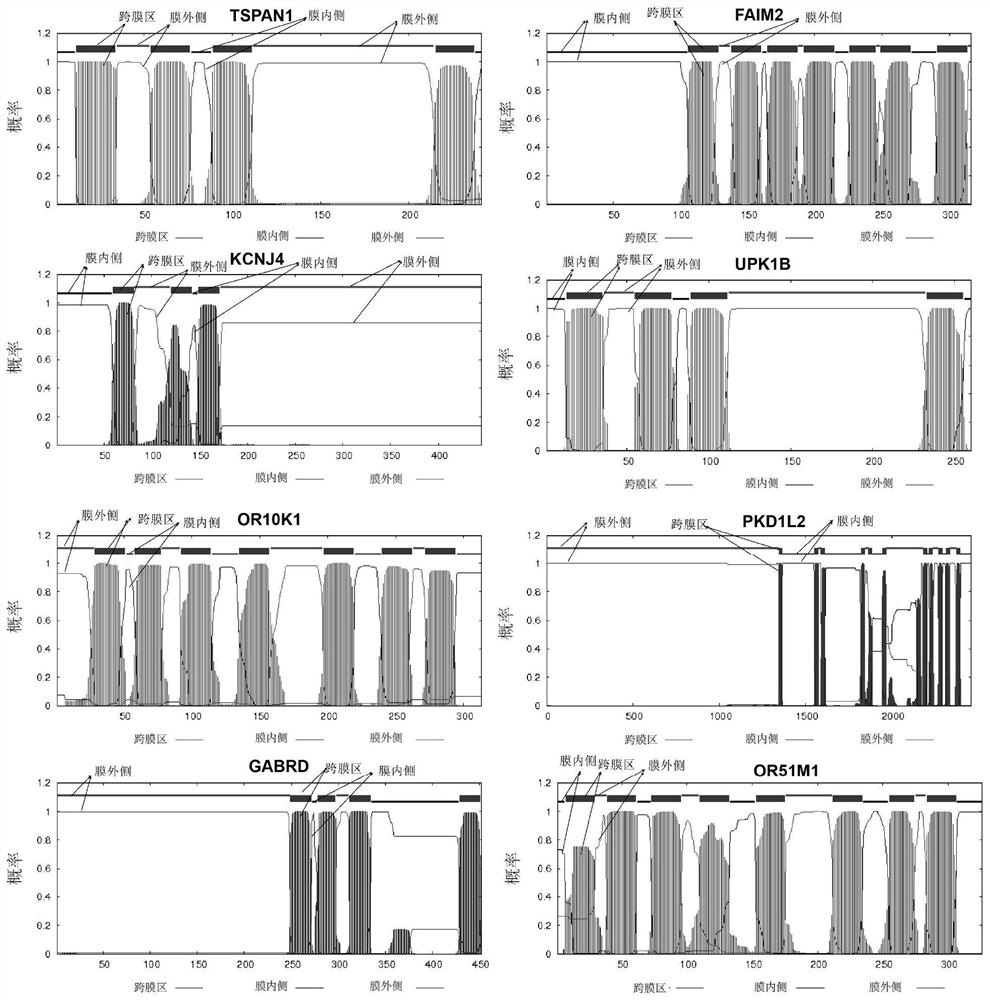
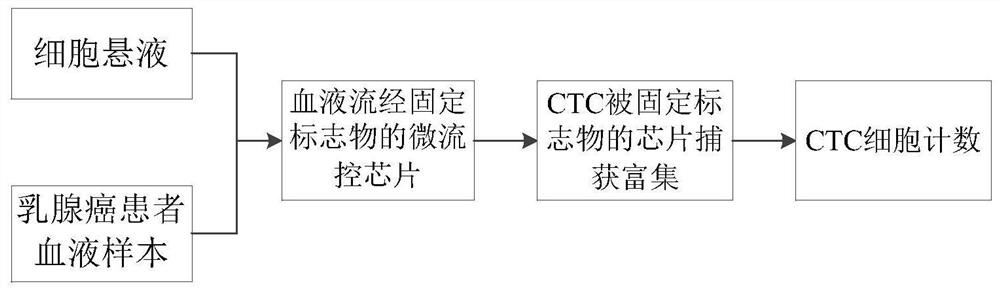
[0047] Prepare or purchase TSPAN1, FAIM2, KCNJ4, GABRD, PDK1L2, OR1OK1, UPK1B and OR51M1 antibodies, respectively coat the chip with these antibodies, and test the ability of each antibody to enrich and screen breast cancer cells (methods such as image 3 shown, excluding blood samples). In this example, classic breast cancer cells SKBR3 and MCF-7 were selected as experimental cells. After coating the chip, the cultured SKBR3 and MCF-7 cells were counted, 1000 cells in each group, flowed through the chip at a certain speed, and the cells captured by the chip were collected with the collection solution and counted. The capture ability of different antibodies on SKBR3 and MCF-7 breast cancer cells was counted. The result is as Figure 4 , the y-axis represents the capture efficiency, that is, the percentage of captured cells enriched by a single antibody. The x-axis represents newly developed breast cancer CTCs specifically expressing surface marker antibodies.
[0048] It c...
Embodiment 2
[0050] Use the screened surface marker antibody combined with EpCam to test the ability of superimposed antibody enrichment to capture breast cancer tumor cells (method such as image 3 shown, excluding blood samples). The result is as Figure 5 As shown, the y-axis represents the capture efficiency, that is, the percentage of antibody-enriched captured cells. The x-axis represents EpCam and its combination with other newly developed surface marker antibodies. The mass ratio of EpCam and corresponding antibody is 1:1.
[0051] The classic breast cancer cells SKBR3 and MCF-7 were selected as experimental cells. After coating the chip, the cultured SKBR3 and MCF-7 cells were counted, 1000 cells in each group, flowed through the chip at a certain speed, and the cells captured by the chip were collected with the collection solution and counted. The capture ability of different antibodies on SKBR3 and MCF-7 breast cancer cells was counted. It can be seen from the figure that t...
Embodiment 3
[0053] Use the screened surface marker antibody combined with HER2 to test the ability of superimposed antibody enrichment to capture breast cancer tumor cells (method such as image 3 shown, excluding blood samples). The result is as Image 6 As shown, the y-axis represents the capture efficiency, that is, the percentage of antibody-enriched captured cells. The x-axis represents HER2 and combinations with other newly developed surface marker antibodies. The mass ratio of HER2 and corresponding antibody is 1:1.
[0054] The classic breast cancer cells SKBR3 and MCF-7 were selected as experimental cells. After coating the chip, the cultured SKBR3 and MCF-7 cells were counted, 1000 cells in each group, flowed through the chip at a certain speed, and the cells captured by the chip were collected with the collection solution and counted. The capture ability of different antibodies on SKBR3 and MCF-7 breast cancer cells was counted. It can be seen from the figure that the comb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com