Composition and application of furandiene-germacrone composition in preparation of psoriasis medicines
A technology of furadienes and compositions, applied in the field of pharmacy, can solve problems such as long course of disease, expensive side effects of drugs, troubles in normal life and daily work of patients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
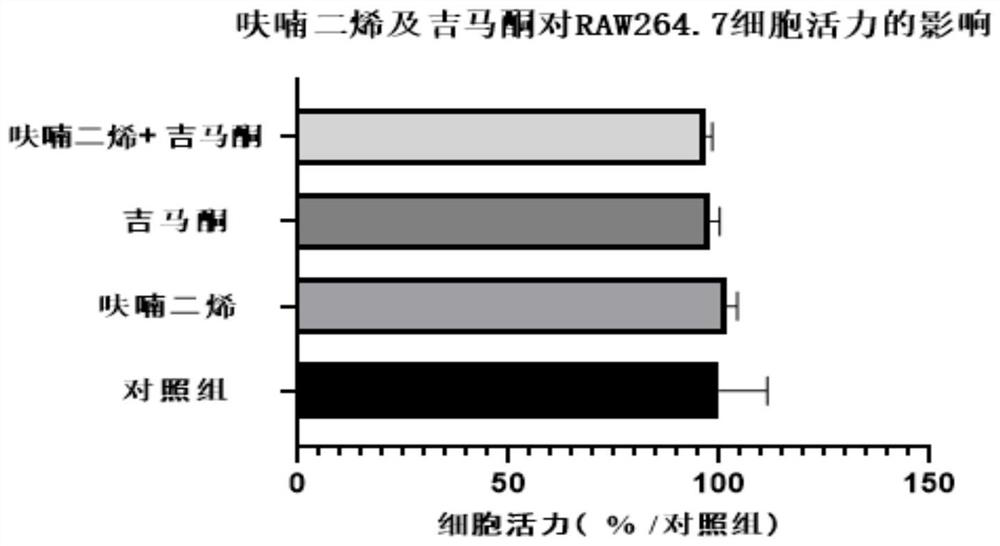
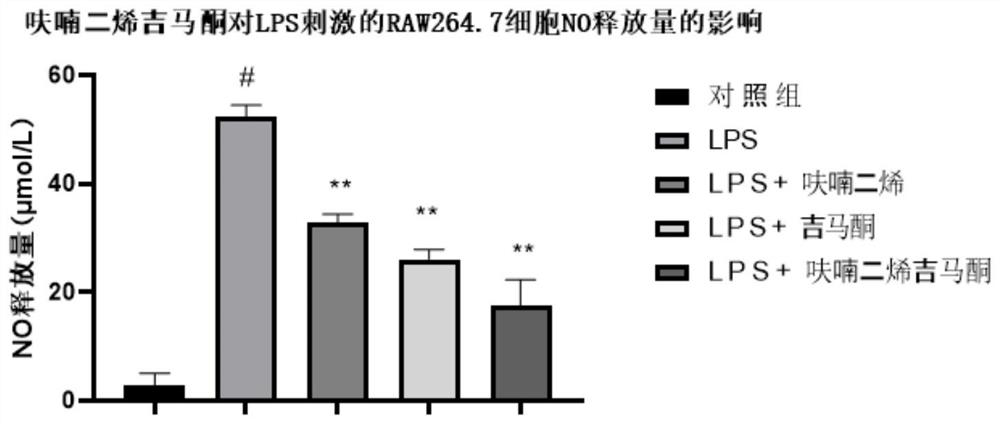
Embodiment 1
[0025] Inhibitory Effect of Furandiene Gemaconone Composition on the Release of Nitric Oxide (NO) from Mouse Mononuclear Macrophages (RAW264.7) Induced by Lipopolysaccharide (LPS)
[0026] 1. Source of materials
[0027] 1. Cell line:
[0028] The RAW264.7 cell line was purchased from Wuhan Punuosai Life Technology Co., Ltd.;
[0029] 2. Main reagents:
[0030] Furanadiene and gemaerone were provided by Hainan Bikai Drug Research Institute; Phosphate BufferedSaline (PBS) buffer, trypsin and penicillin-streptomycin solution were purchased from Gibco Company; RAW264.7 cell special medium and fetal calf Serum was purchased from Wuhan Punuosai Life Technology Co., Ltd.; lipopolysaccharide (LPS) was purchased from Shanghai Yuanye Biotechnology Co., Ltd.; CCK-8 kit was purchased from Glpbio; nitric oxide (NO) detection kit was purchased from Biyuntian Biotechnology Co., Ltd;
[0031] 2. Experimental method
[0032] 1. Cell culture
[0033] RAW264.7 cells were grown in RAW264.7...
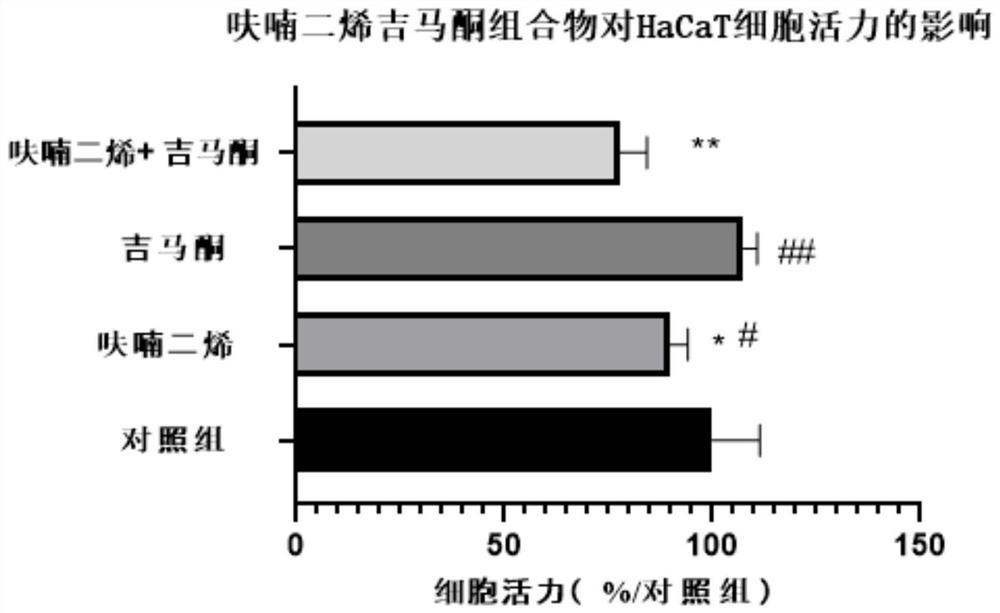
Embodiment 2
[0050] Example 2 Inhibitory effect of the furadiened gemaerone composition on the viability of human immortalized epidermal cell lines (HaCaT)
[0051] 1. Source of materials
[0052] 1. Cell line:
[0053] Immortalized human epidermal cell line (HaCaT) was purchased from Wuhan Punosai Life Technology Co., Ltd.
[0054] 2. Main reagents:
[0055] Furanadiene and Gemaerone were provided by Effective Company of Hainan Bikai Pharmaceutical Research Institute; Phosphate BufferedSaline (PBS) buffer solution, Dulbecco's Modified Eagle Medium (DMEM) medium, trypsin and penicillin-streptomycin solution were purchased from Gibco Company; The CCK-8 kit was purchased from Glpbio;
[0056] 2. Experimental method
[0057] 1. Cell culture
[0058] HaCaT cells were grown in DMED medium containing 15% fetal bovine serum, 100 U / ml penicillin and 100 mg / l streptomycin at 37°C, 5% CO 2 cultured in an incubator. The cells were passaged and digested with 0.25% trypsin containing 0.02% EDTA, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com