Preparation method of tumor neoantigen specific T cells
A specific and cell-based technology, applied in the field of cell biology, can solve the problems of low proportion of tumor neoantigen-specific T cells, limited killing effect of tumor cells, and low purity of mature DC cells, so as to achieve strong specific immune response and good stimulation Immune system, effect of improving phagocytosis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
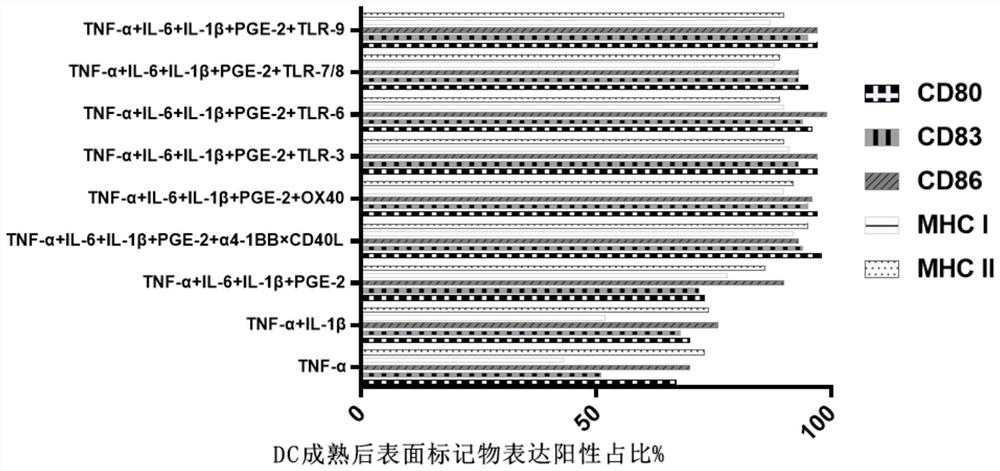
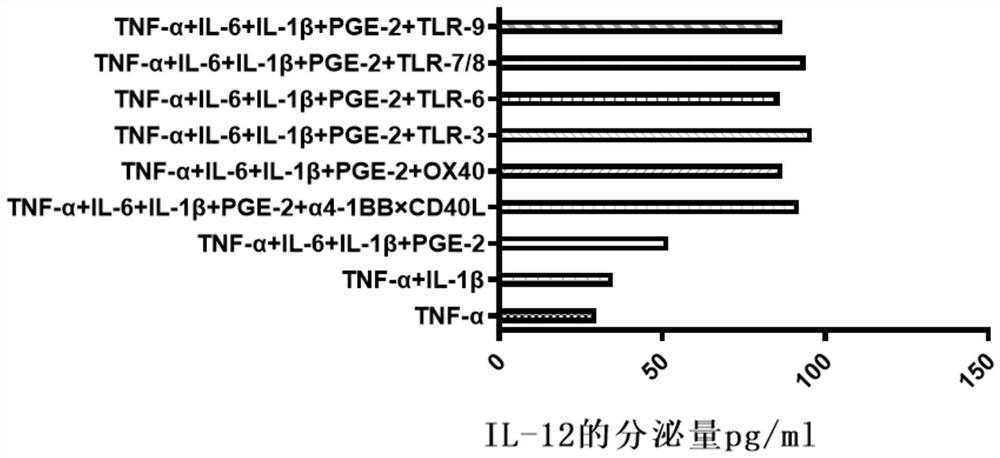
[0053] A method for preparing tumor neoantigen-specific T cells and verifying their effectiveness in killing tumor cells, comprising:
[0054] Step 1, synthesizing tumor neoantigen polypeptide;
[0055] For specific methods, please refer to a screening method for individualized tumor neoantigen peptides in patent 202110251383.X to obtain polypeptide sequences, and then synthesize polypeptides, and use solid-phase synthesis technology to produce suitable purity polypeptides; to verify the immune function of the obtained polypeptides:
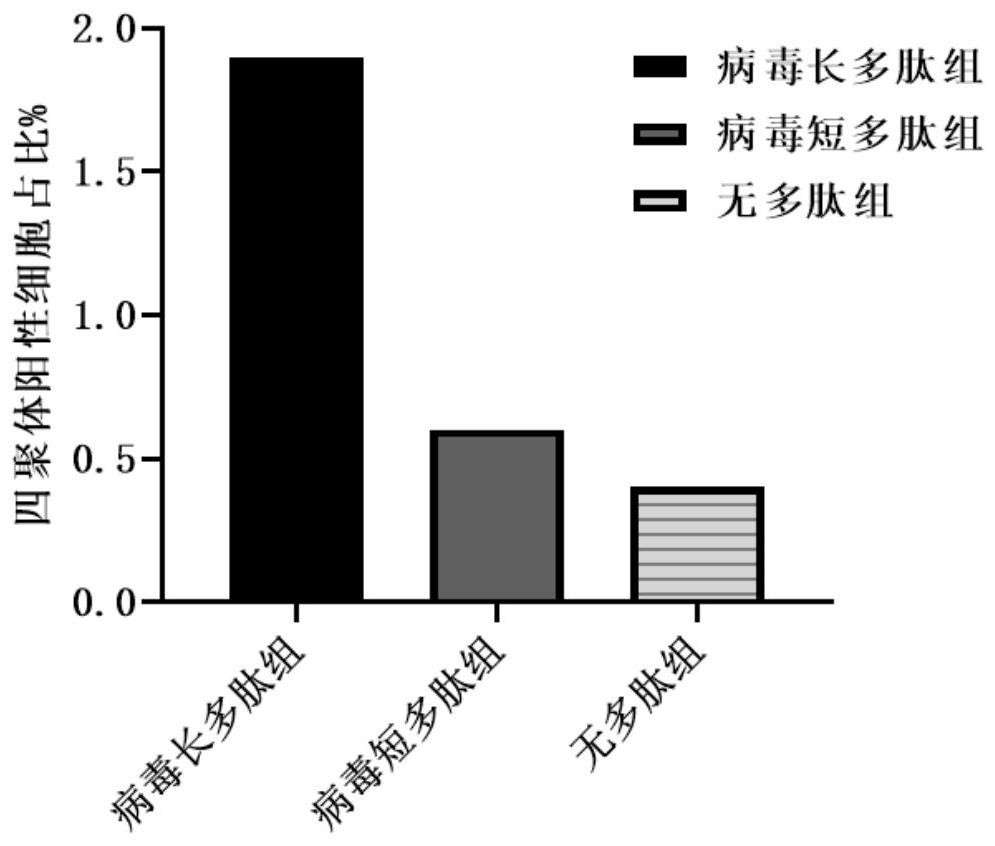
[0056] Blood samples from healthy volunteers were drawn to separate PBMCs, and the immune function of the peptide vaccine was verified in vitro, and the secretion of IFN-γ after peptide stimulation was detected. Using the ELISpot method, with the polypeptide vaccine as the stimulus, the long polypeptide of the present invention as the experimental group 1, the corresponding short polypeptide at the same site in the long polypeptide as the experim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com