Antibacterial peptide RLR derived from sheep source as well as preparation method and application thereof
An antibacterial peptide, Gram-negative bacteria technology, applied in the direction of cationic antibacterial peptides, antibacterial drugs, chemical instruments and methods, etc. Action, the effect of narrowing the antibacterial spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
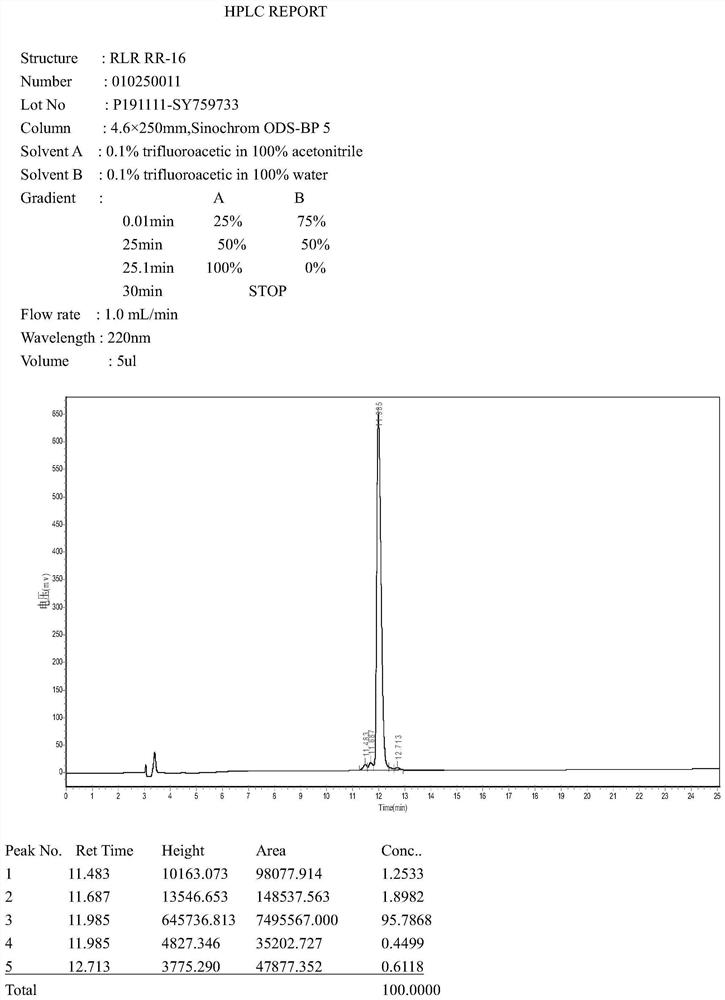
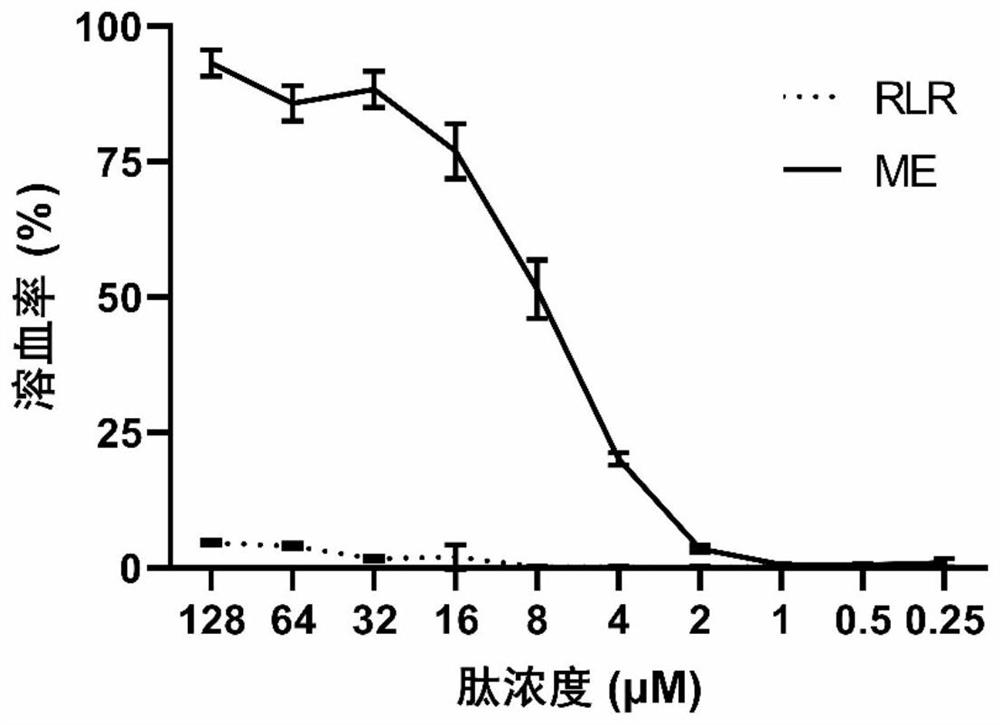
Image
Examples
Embodiment 1
[0014] Design of Antimicrobial Peptides
[0015] The amino acid sequence of the goat-derived antimicrobial peptide ChBac5 is:
[0016] RFRPPIRRPPIRPPFNPPFRPPVRPPFRPPFRPPFRPPIGPFP
[0017] By intercepting 16 amino acids at the end of the goat-derived antimicrobial peptide ChBac5, a polypeptide RFR containing 5 positive charges and a hydrophobic value of 3.66 was obtained, and its amino acid sequence was: RFRPPIRRPPPIRPPFN;
[0018] Replace the double Pro-Pro of the RFR rigid structure with Ala that promotes α-helix, and replace the terminal Asn with Arg to further increase the positive charge content of the antimicrobial peptide, and obtain a polypeptide containing 6 positive charges and a hydrophobic value of 0.186 RFRR, its amino acid sequence is: RFRAAIRRAAIRAAFR;
[0019] Ile and Lue were used to replace the double Ala-Ala on both sides to increase hydrophobicity, and Lue was used to replace Phe to reduce cytotoxicity, and finally the C-terminal was amidated to increase o...
Embodiment 2
[0023] Determination of antimicrobial activity of antimicrobial peptides
[0024] 1. Determination of antibacterial activity: the minimum inhibitory concentration of the antimicrobial peptide RLR was determined by the micro broth dilution method. Using 2 mg / ml BSA (containing 0.01% acetic acid) as the diluent, a series of gradient antimicrobial peptide solutions were sequentially prepared using the double dilution method. Take 100 μL of the above solution and place it in a 96-well cell culture plate, then add an equal volume of the bacteria solution to be tested (~10 5 individual / mL) in each well. Positive controls (containing bacterial fluid but not antimicrobial peptides) and negative controls (neither bacterial fluid nor antimicrobial peptides) were set up. Incubate at a constant temperature of 37°C for 14-18h, and use a microplate reader at 492nm (OD 492nm ) to determine the minimum inhibitory concentration. The test results are shown in Table 2.
[0025] Antibacteria...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com