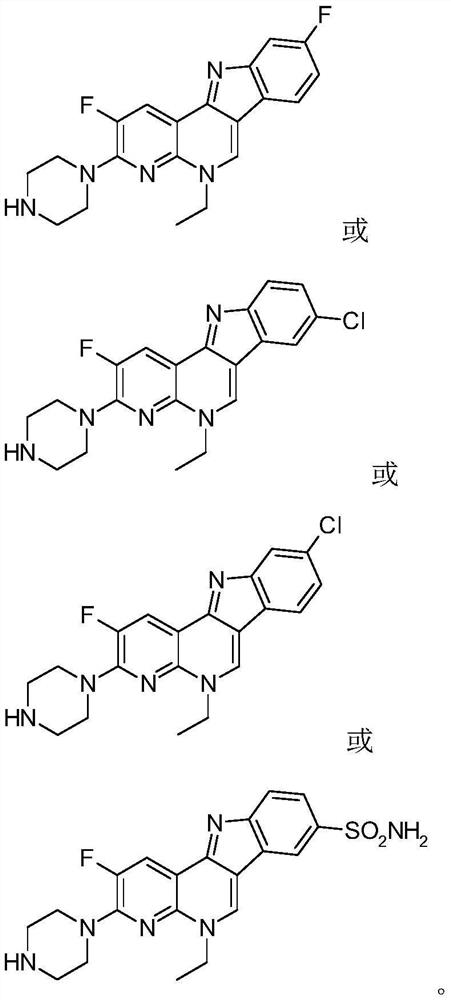
Isorcryptolepine analogue prepared by taking enoxacin as raw material as well as preparation method and application of iso-cryptolepine analogue
A technology of isoberaline and enoxacin, applied in organic chemistry, antibacterial drugs, etc., can solve the problems of poor water solubility, difficult source of albino alkaloids, low bioavailability, etc., to increase penetration Function, excellent in vitro growth inhibition activity of Mycobacterium tuberculosis, effect of improving water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
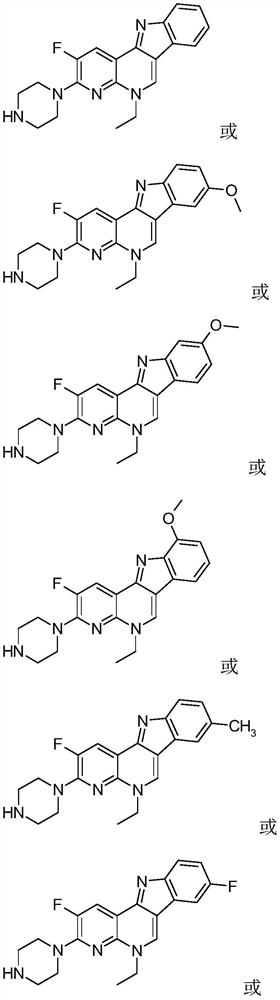
[0035] 2-fluoro-3-piperazin-1-yl-5-ethyl-5H-indolo[3,2-c][1,8]naphthyridine (I-1), its chemical structure is:
[0036]
[0037] That is, R in formula I is an H atom.
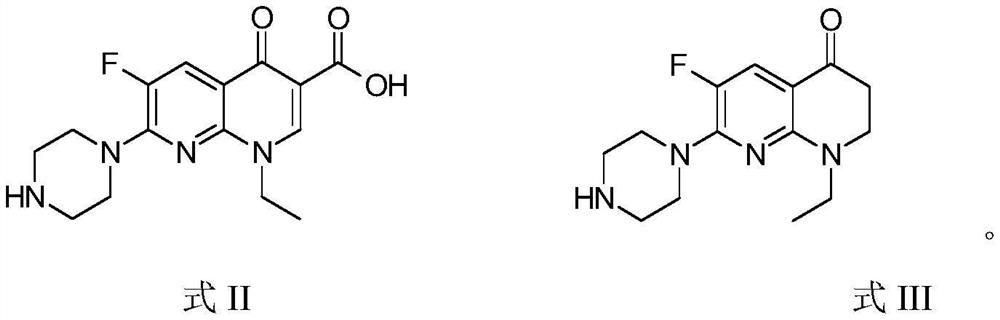
[0038] The preparation method of the compound (I-1) is: take 6-fluoro-1-ethyl-7-piperazin-1-yl-2,3-dihydro-[1,8]naphthyridine-4(1H) - Dissolve 1.0 g (3.6 mmol) of ketone III in 15 mL of absolute ethanol, add 0.50 g (4.6 mmol) of phenylhydrazine, stir and react at room temperature for 20 h (the disappearance of raw material III was observed by TLC), and a large amount of precipitates are formed. Concentrated hydrochloric acid (0.50 mL) was added as a cyclization catalyst, and the mixed reactants were refluxed for 20 h and left overnight (12 h, the same below). Collect the resulting solid by filtration, dissolve the solid with 50 mL of deionized water, add an appropriate amount of activated carbon, and reflux for 1 h for decolorization. Filtrate hot, and adjust the pH of the filtrate to ≈10.0 with ammonia wat...
Embodiment 2
[0040] 2-fluoro-8-methoxy-3-piperazin-1-yl-5-ethyl-5H-indolo[3,2-c][1,8]naphthyridine (I-2), which The chemical structural formula is:
[0041]
[0042] That is, R in formula I is methoxy.
[0043] The preparation method of this compound (I-2) is: take 6-fluoro-1-ethyl-7-piperazin-1-yl-2,3-dihydro-[1,8]naphthyridine-4(1H) - Dissolve 1.0 g (3.6 mmol) of ketone III in 15 mL of absolute ethanol, add 0.62 g (4.5 mmol) of p-methoxyphenylhydrazine, stir and react at room temperature overnight (the disappearance of raw material III was observed by TLC), and an obvious precipitate is formed. Concentrated hydrochloric acid (0.50 mL) was added, and the mixed reactants were refluxed for 16 h and left overnight. Collect the resulting solid by filtration, dissolve the solid with 50 mL of deionized water, add an appropriate amount of activated carbon, and reflux for decolorization for 1 h. Filtrate hot, and adjust the pH of the filtrate to ≈10.0 with ammonia water. The resulting soli...
Embodiment 3
[0045] 2-fluoro-9-methoxy-3-piperazin-1-yl-5-ethyl-5H-indolo[3,2-c][1,8]naphthyridine (I-3), Its chemical structural formula is:
[0046]
[0047] That is, R in formula I is methoxy.
[0048] The preparation method of this compound (I-3) is: take 6-fluoro-1-ethyl-7-piperazin-1-yl-2,3-dihydro-[1,8]naphthyridine-4(1H) - Dissolve 1.0 g (3.6 mmol) of ketone III in 15 mL of absolute ethanol, add 0.83 g (6.0 mmol) of m-methoxyphenylhydrazine, stir and react at room temperature for 24 hours (the disappearance of raw material III was observed by TLC), and an obvious precipitate is formed. Concentrated hydrochloric acid (0.50 mL) was added, and the mixed reactant was refluxed for 16 h and left overnight. Collect the resulting solid by filtration, dissolve the solid with 50 mL of deionized water, add an appropriate amount of activated carbon, and reflux for 1 h for decolorization. Filtrate hot, and adjust the pH of the filtrate to ≈10.0 with ammonia water. The resulting solid was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com