Synthesis method of 1-fluoronaphthalene
A synthetic method and technology of fluoronaphthalene, applied in the field of synthesis of 1-fluoronaphthalene, capable of solving the problems of low yield of 1-fluoronaphthalene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
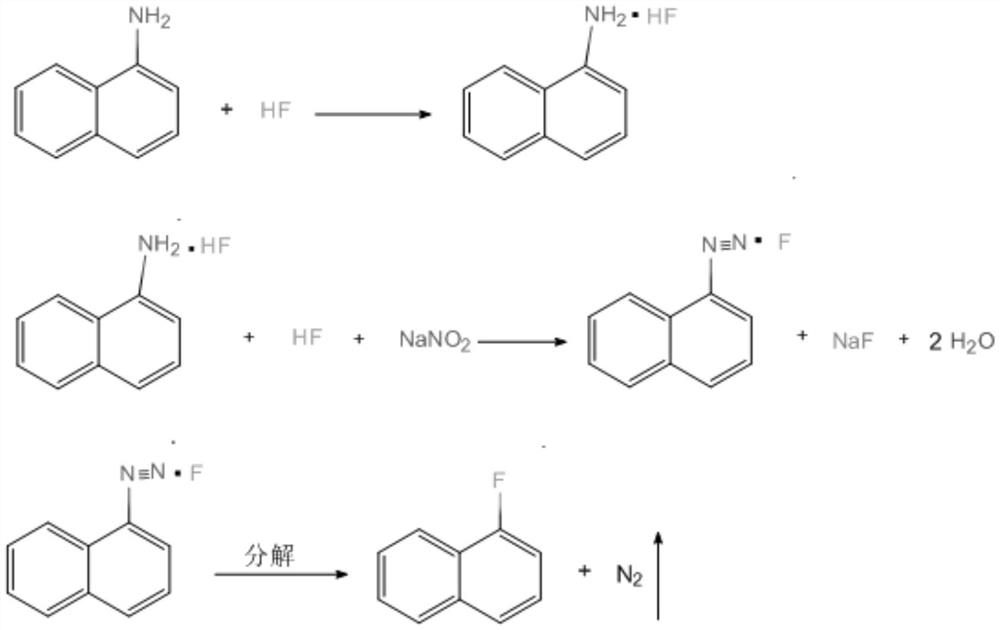
[0019] This specific embodiment provides a kind of synthetic method of 1-fluoronaphthalene, comprises the following steps:
[0020] S1, adding menaphthylamine to anhydrous hydrofluoric acid below 10°C to form menaphthylamine hydrofluoride; the mass ratio of said anhydrous hydrofluoric acid to said menaphthylamine is (2-5):1 ;
[0021] S2. Add anhydrous nitrite to the menaphthylamine hydrofluoride, and perform a diazotization reaction below 5°C to obtain an α-diazonaphthyl hydrofluoride solution; the anhydrous nitrate and the The mass ratio of menaphthylamine hydrofluoride is 1:(1.8-2); Further, described anhydrous nitrite is anhydrous sodium nitrite or anhydrous potassium nitrite;
[0022] S3. Thermally decomposing the α-diazonaphthalene hydrofluoride solution in a thermal decomposer at 50-80° C. to obtain a solution containing 1-fluoronaphthalene;
[0023] S4. Layering the solution containing 1-fluoronaphthalene to obtain a lower organic layer and an upper acid layer, neutr...
Embodiment 1
[0028] The present embodiment proposes a kind of synthetic method of 1-fluoronaphthalene, comprises the following steps:
[0029] S1, adding menaphthylamine to anhydrous hydrofluoric acid at 5°C to form menaphthylamine hydrofluoride; the mass ratio of said anhydrous hydrofluoric acid to said menaphthylamine is 3:1; menaphthylamine It is a block solid below 50°C, so menaphthylamine needs to be crushed into powder and then fed with an auger;
[0030] S2. Add anhydrous sodium nitrite to the menaphthylamine hydrofluoride, and perform a diazotization reaction at 5° C. to obtain an α-diazonaphthyl hydrofluoride solution; the anhydrous nitrate and the The mass ratio of menaphthylamine hydrofluoride is 1:1.8; Anhydrous nitrite is solid, therefore adopts auger to continuously and slowly wring in menaphthylamine hydrofluoride;
[0031] S3. Thermally decompose the α-diazonaphthalene hydrofluoride solution in a thermal decomposer at 50° C. to obtain a solution containing 1-fluoronaphthal...
Embodiment 2
[0034] The present embodiment proposes a kind of synthetic method of 1-fluoronaphthalene, comprises the following steps:
[0035] S1, adding menaphthylamine to anhydrous hydrofluoric acid at 8°C to form menaphthylamine hydrofluoride; the mass ratio of said anhydrous hydrofluoric acid to said menaphthylamine is 2:1; menaphthylamine It is a block solid below 50°C, so menaphthylamine needs to be crushed into powder and then fed with an auger;
[0036] S2. Add anhydrous sodium nitrite to the menaphthylamine hydrofluoride, and perform a diazotization reaction below 3°C to obtain an α-diazonaphthyl hydrofluoride solution; the anhydrous nitrate and the The mass ratio of menaphthylamine hydrofluoride is 1:2; Anhydrous nitrite is solid, so adopts auger to continuously slowly wring in menaphthylamine hydrofluoride;
[0037] S3. Thermally decompose the α-diazonaphthalene hydrofluoride solution in a thermal decomposer at 60°C to obtain a solution containing 1-fluoronaphthalene; at this t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com