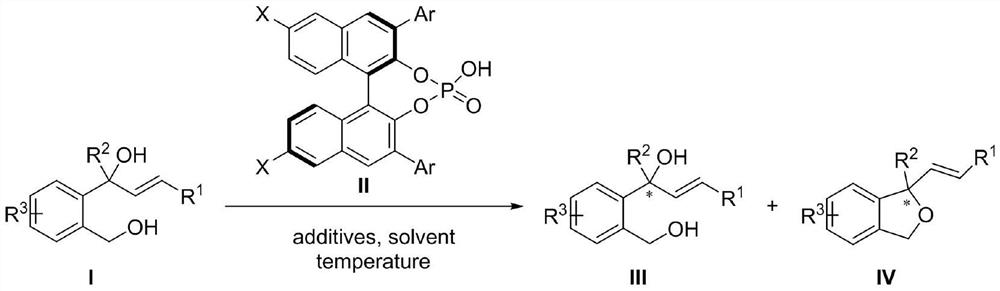
Kinetic resolution method of aryl allyl tertiary alcohol catalyzed by chiral phosphoric acid
A technology of aryl allyl tertiary alcohol and aryl allyl tertiary alcohol, which is applied in the field of dynamic resolution of aryl allyl tertiary alcohol catalyzed by chiral phosphoric acid, can solve the problem of catalytic asymmetric synthesis, which is less reported, Loss of stereochemical integrity, difficulty in splitting reactions, etc., achieve good industrial application prospects, avoid the use of transition metals, and have a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1: the synthesis of product III-1 and IV-1
[0062]
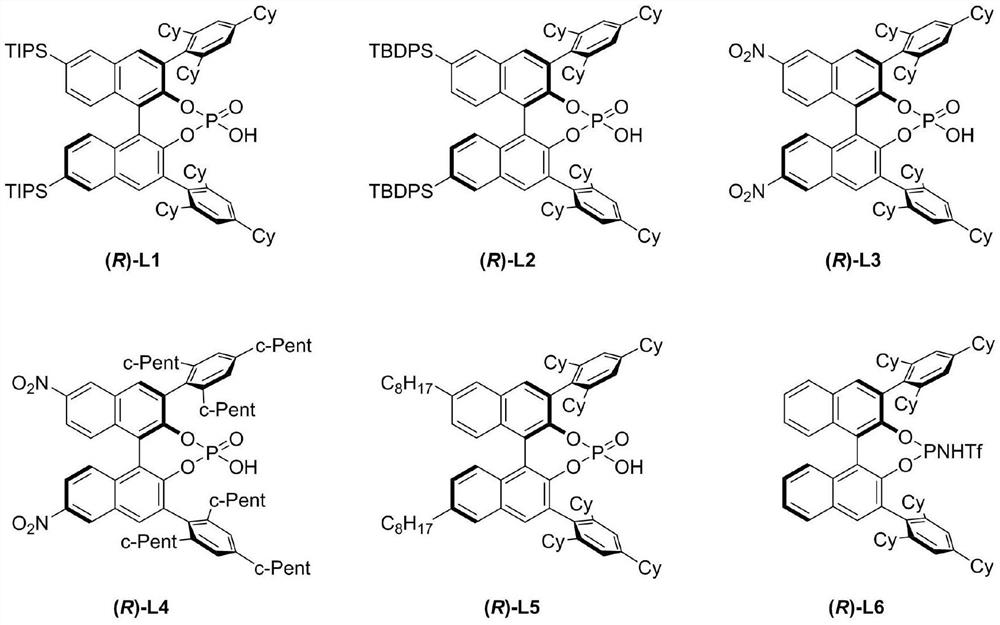
[0063] Experimental procedure: in N 2 Under protection, the reaction system was sealed for anhydrous and oxygen-free treatment, and the temperature was adjusted to 0°C. In a 10mL reaction flask, I-1 (0.1mmol, 1.0equiv) and chiral binaphthol catalyst (R)- L1 (0.015mmol, 0.15equiv), additives Molecular sieves (160 mg) and a solvent of chloroform (0.5 mL) were used for the reaction. The reaction was monitored by HPLC analysis until the reaction was completed, the reaction system was filtered through diatomaceous earth and concentrated, and the concentrated crude product was separated and purified by column chromatography, and the eluent was petroleum / ethyl acetate=5 / 1 (v / v) , the target compound III-1 was obtained as a white foamy solid with a yield of 47% and an ee value of 92%. [α] D 20 =–17.28 (c 1.0, CHCl 3 ) 1 H NMR (600MHz, DMSO-d 6 )δ7.56(d, J=7.6Hz, 1H), 7.45(dd, J=7.8, 1.8Hz, 3H), 7.35–7....
Embodiment 2
[0073] Embodiment 2: the synthesis of product III-2 and IV-2
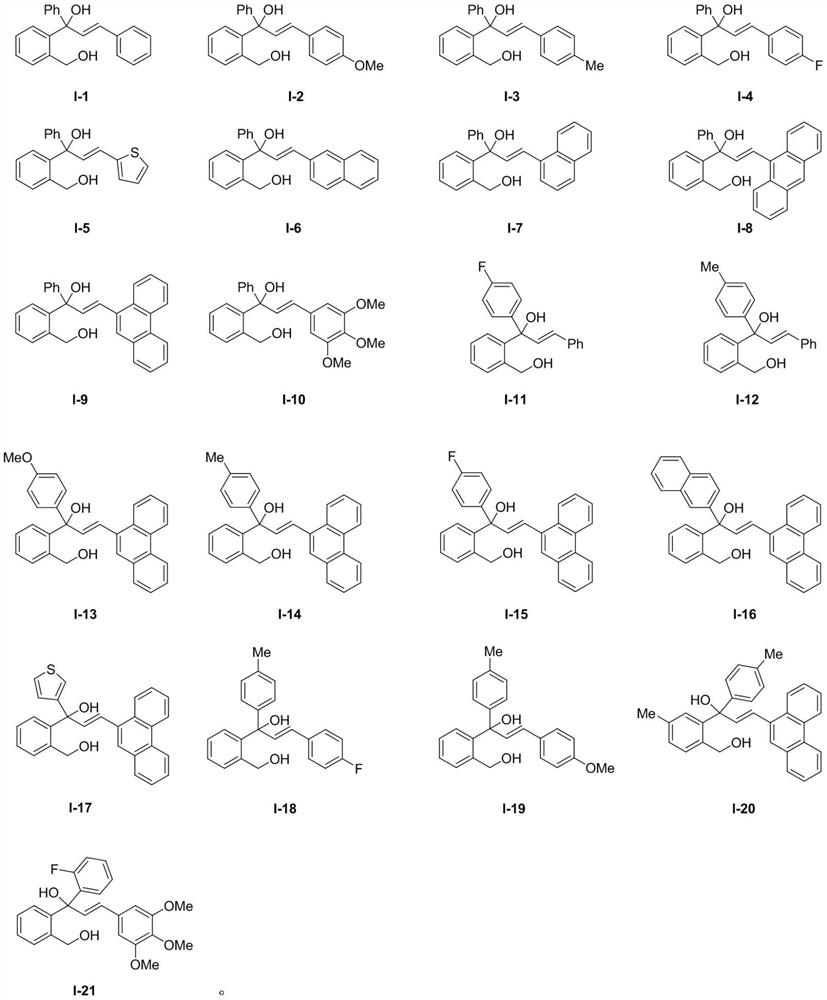
[0074] The compound shown in Formula I-1 in Example 1 is replaced by the compound shown in Formula I-2 in equivalent molar amounts, and all the other operating steps are the same as in Example 1 to finally obtain the corresponding compounds III-2 and IV-2 .
[0075]
[0076] The product III-2 was obtained as a white foamy solid with a yield of 42% and an ee value of 93%. [α] D 20 =–5.29 (c1.0, CHCl 3 ). 1 H NMR (400MHz, DMSO-d 6 )δ7.55(d, J=7.0Hz, 1H), 7.42(dd, J=12.4, 4.4Hz, 3H), 7.36–7.27(m, 3H), 7.27–7.15(m, 4H), 6.91(d ,J=16.0Hz,1H),6.87(d,J=8.8Hz,2H),6.39(d,J=16.0Hz,1H),6.20(s,1H),5.05(t,J=5.4Hz,1H ),4.46(dd,J=14.4,5.4Hz,1H),4.16(dd,J=14.4,5.2Hz,1H),3.73(s,3H). 13 C NMR (101MHz, DMSO-d 6 )δ157.8, 143.6, 141.1, 138.3, 137.2, 136.7, 128.6, 128.0, 127.4, 127.4, 127.2, 127.1, 126.6, 126.4, 125.9, 113.2, 79.2, 78.4, 61.0, 55.0, 39.3, 3.95, 39.9 ,39.1,38.9.HRMS(ESI)m / z calcd.for C 23 h 22 NaO 3 [M+Na...
Embodiment 3
[0078] Embodiment 3: the synthesis of product III-3 and IV-3
[0079] The compound shown in Formula I-1 in Example 1 is replaced by the compound shown in Formula I-3 in equivalent molar amounts, and all the other operating steps are the same as in Example 1 to finally obtain the corresponding compounds III-3 and IV-3 .
[0080]
[0081] The product III-3 was obtained as a white foamy solid with a yield of 30% and an ee value of 70%. [α] D 20 =–7.19 (c1.0, CHCl 3 ). 1 H NMR (400MHz, DMSO-d 6 )δ7.55(d, J=7.4Hz, 1H), 7.44(dd, J=7.6, 1.2Hz, 1H), 7.39–7.28(m, 7H), 7.29–7.18(m, 2H), 7.13(d ,J=8.0Hz,2H),6.86(d,J=16.0Hz,1H),6.38(d,J=16.0Hz,1H),6.28(s,1H),5.06(t,J=5.4Hz,1H ),4.46(dd,J=14.4,5.4Hz,1H),4.11(dd,J=14.4,5.2Hz,1H),2.28(s,3H). 13 C NMR (101MHz, DMSO-d 6 )δ146.5,143.6,141.1,136.7,136.0,133.8,129.2,128.0,127.8,127.3,127.2,126.7,126.5,126.4,126.2,125.9,78.7,60.9,20.8.HRMS(ESI)m / z C calcd 23 h 22 NaO 2 [M+Na] + :353.1620; found 353.1512.
[0082] The product IV-3 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com