Antibacterial peptide hrNCM as well as preparation method and application thereof
A technology for solid-phase synthesis of antimicrobial peptides and polypeptides, applied in the field of biomedicine, can solve the problems of high cytotoxicity, low antibacterial activity, and poor stability, and achieve the effects of low hemolytic activity, small molecular weight, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Chemical Synthesis of the Antimicrobial Peptide hrNCM from the Transformed Green Sea Turtle
[0020] The green sea turtle antimicrobial peptide Cm-CATH2 is a polypeptide encoded by a gene, containing 33 amino acid residues, a molecular weight of 4089.9Da, and an isoelectric point of 12.96. The full sequence of the green sea turtle antimicrobial peptide Cm-CATH2 is: Arg 1 Arg 2 Ser 3 Arg 4 Phe 5 Gly 6 Arg 7 Phe 8 Phe 9 Lys 10 Lys 11 Val 12 Arg 13 Lys 14 Gln 15 Leu 16 Gly 17 Arg 18 Val 19 Lys 20 Arg 21 His 22 Ser 23 Arg 24 Ile 25 Thr 26 Val 27 Gly 28 Gly 29 Arg 30 met 31 Arg 32 Phe 33 (SEQ ID NO. 1). According to the amino acid sequence of the green sea turtle antimicrobial peptide Cm-CATH2, a series of peptide chain shortening peptides were designed by molecular modification methods, and the modified N-CM4 was screened out through research on antibacterial, anti-inflammatory and cytotoxic hemolytic activities, ...
Embodiment 2
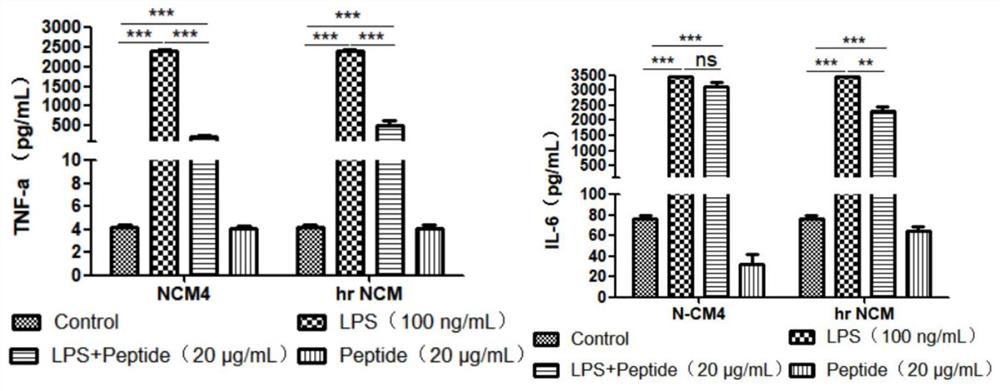
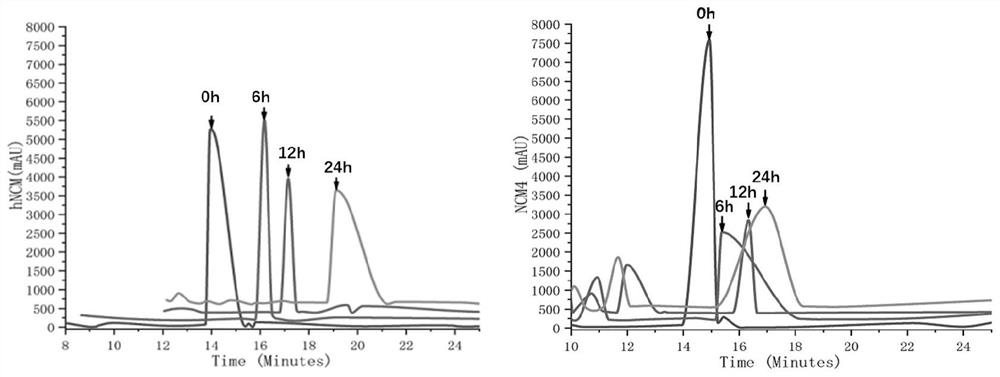
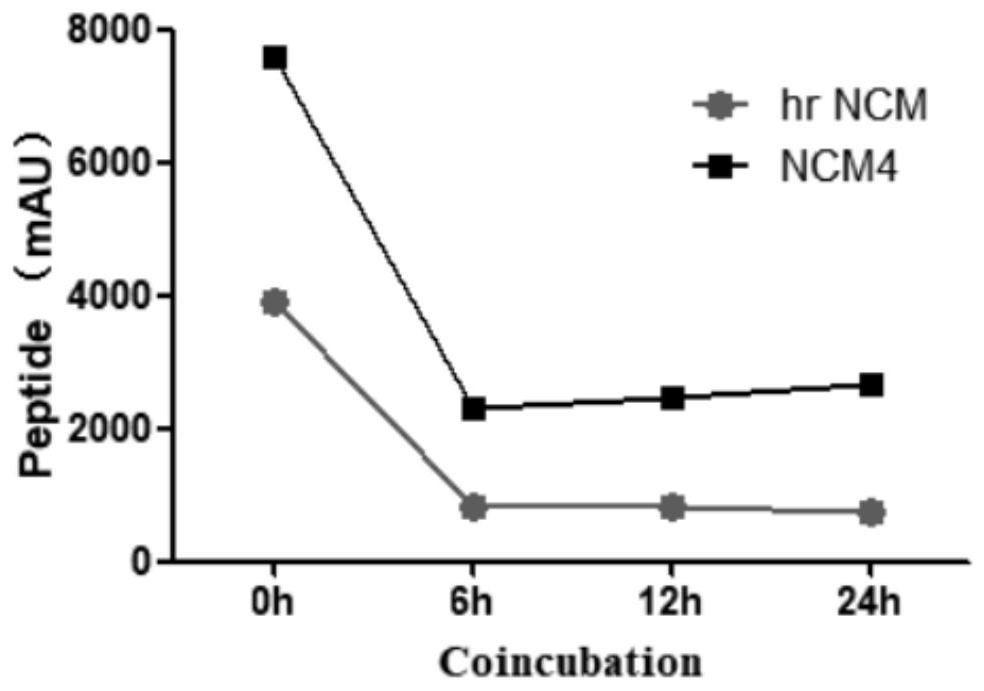
[0028] hrNCM pharmacological experiments:
[0029] 1. Determination of antibacterial activity of hrNCM:
[0030] (1) Pick the test strains preserved on the slant and spread them evenly on the MH solid culture medium (Beijing Suo Laibao Technology Co., Ltd.) plate, place the sterilized 0.5cm diameter filter paper on the surface of the culture medium, Add 10 μl of 2 mg / ml antibacterial peptide hrNCM sample solution dissolved in sterilized deionized water dropwise, incubate upside down at 37°C for 18-20 hours, and observe whether the antibacterial zone is formed or not. If the sample has antibacterial activity, a clear and transparent bacteriostatic zone will be formed around the filter paper, and the larger the bacteriostatic zone, the stronger the antibacterial activity of the sample.
[0031] (2) Determination of Antimicrobial Peptide hrNCM Minimum Inhibitory Concentration (2-fold dilution method):
[0032] Select the strains with the inhibition zone in the previous experime...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com