Fluoketamine artificial hapten, artificial antigen and preparation method and application thereof
A technology of artificial hapten and artificial antigen, which is applied in the field of artificial antigen and its preparation, fluoroamine artificial hapten, can solve the problems of expensive equipment and time-consuming detection, and achieve high affinity, strong specificity, accurate immune detection and immune The effect of the analysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
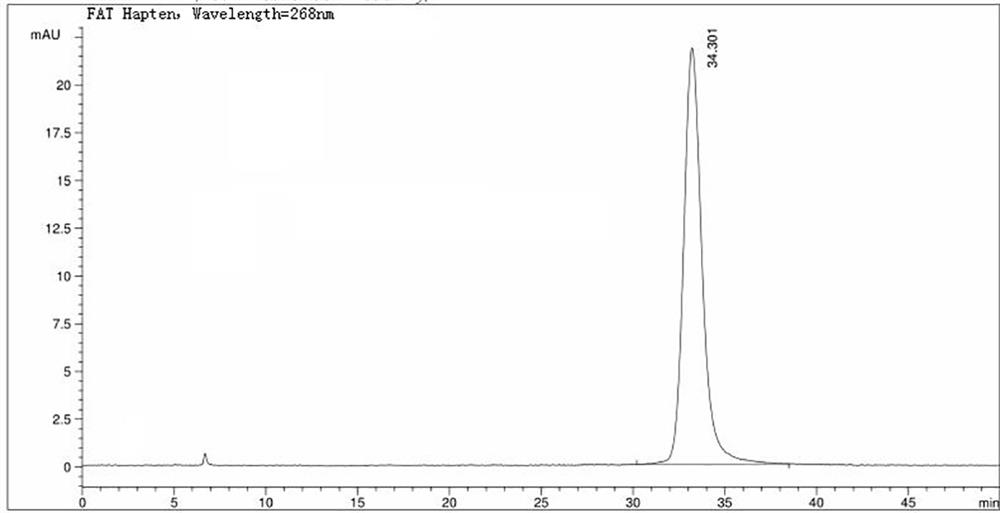
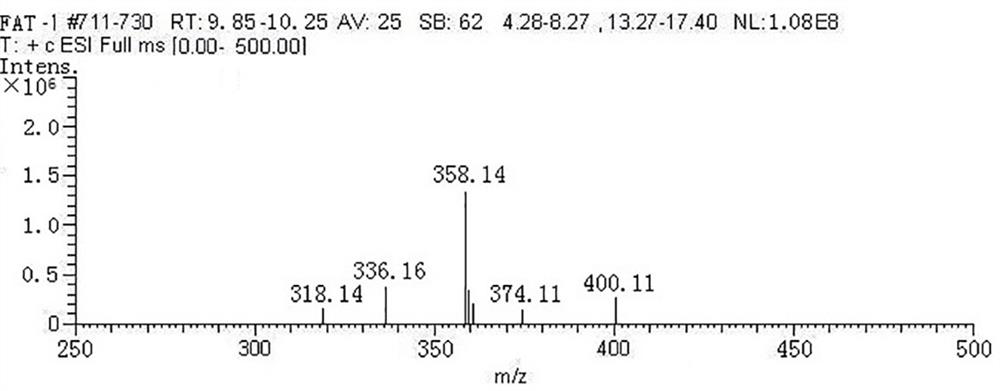
Image
Examples
Embodiment 1
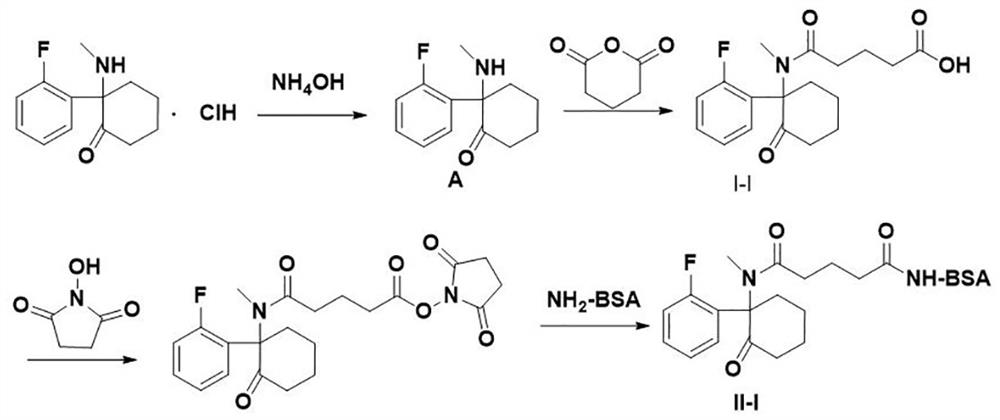
[0066] The implementation of a preparation method of fluoroamine artificial antigen (reaction process as follows figure 1 ), including the following steps:
[0067] Preparation of artificial haptens:
[0068] ① Weigh 200mg (0.777mmol) of fluoroquinone hydrochloride and dissolve it in 20ml of purified water, add ammonia water drop by drop to adjust the pH of the solution to 9. At this time, the solution is a white turbid liquid, extract it with 20ml*3 dichloromethane, and collect the organic phase, dried with anhydrous magnesium sulfate, filtered, and evaporated to dryness under reduced pressure to obtain 165mg (0.747mmol) of colorless oil A;
[0069] The colorless oil A was detected by TLC, the chromatographic solution was ethyl acetate:petroleum ether=1:1 (v / v), the product R f =0.3.
[0070] ② Dissolve 165mg (0.747mmol) of the colorless oily substance A in the previous step in 5ml of pyridine, add a stir bar, then add 170mg (1.491mmol) glutaric anhydride, stir and reflux ...
Embodiment 2
[0084] The implementation of a preparation method of fluoroamine artificial antigen (reaction process as follows Figure 5 ), including the following steps:
[0085] (1) Preparation of artificial hapten of fluoroamine:
[0086] ①Same as Example 1.
[0087] ②Dissolve 183mg (0.826mmol) of the colorless oily substance A in the previous step in 5ml of pyridine, add a stirrer, then add 82.6mg (0.826mmol) of succinic anhydride, stir and reflux in an oil bath at 90°C for 24 hours;
[0088] After the reaction was completed, the solvent was evaporated to dryness under reduced pressure, and azeotroped with 10 ml of absolute ethanol. Add 30ml of dichloromethane to dissolve the residue, then wash the organic phase with 20ml*3 purified water, collect the organic phase, dry with anhydrous magnesium sulfate, filter, and evaporate to dryness under reduced pressure to obtain a brown-black oily substance, which is thin-layer chromatographed (chromatographic liquid is Dichloromethane: 95% eth...
Embodiment 3
[0093] The implementation of a preparation method of fluoroamine artificial antigen (reaction process as follows Figure 6 ), including the following steps:
[0094] (1) Preparation of artificial hapten of fluoroamine:
[0095] ①Same as Example 1.
[0096] ②Dissolve 173mg (0.782mmol) of the colorless oily substance A in the previous step in 5ml of pyridine, add a stirrer, then add 300mg (2.346mmol) of adipic anhydride, stir and reflux in an oil bath at 110°C for 15 hours;
[0097] After the reaction was completed, the solvent was evaporated to dryness under reduced pressure, and azeotroped with 10 ml of absolute ethanol. Add 30ml of dichloromethane to dissolve the residue, then wash the organic phase with 20ml*3 purified water, collect the organic phase, dry with anhydrous magnesium sulfate, filter, and evaporate to dryness under reduced pressure to obtain a brown-black oily substance, which is thin-layer chromatographed (chromatographic liquid is Dichloromethane: 95% ethan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com