Peiminine derivative and preparation method and application thereof
A technology of peimonin B derivatives, applied in the field of chemical structure and synthesis of peiminin B derivatives, can solve the problems of low bioavailability, insoluble in water, etc., achieve simple preparation method, high conversion rate, The effect of less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
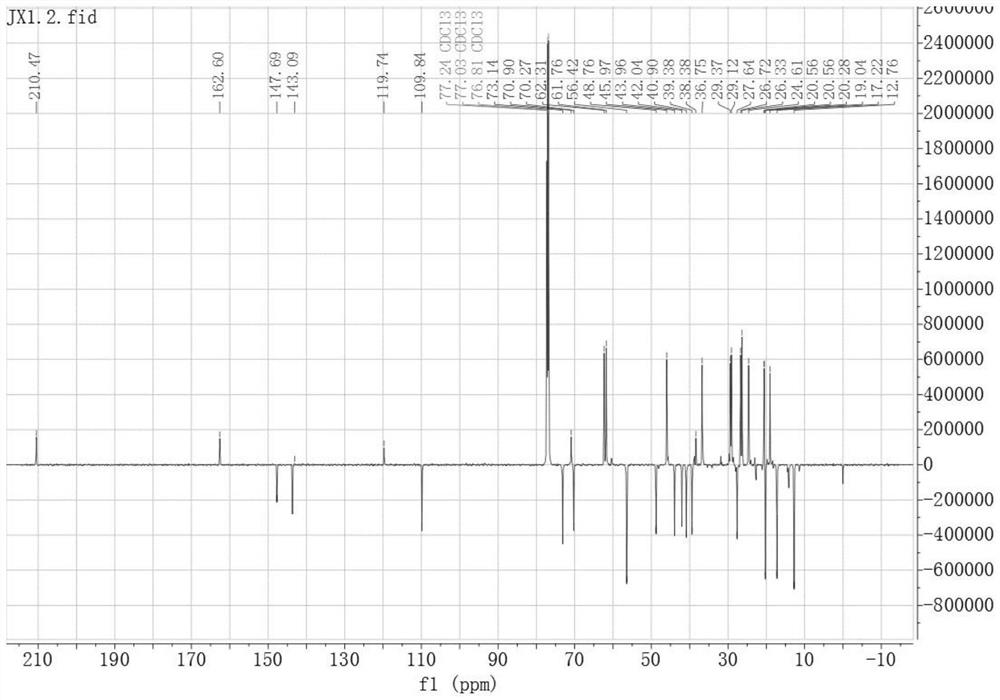
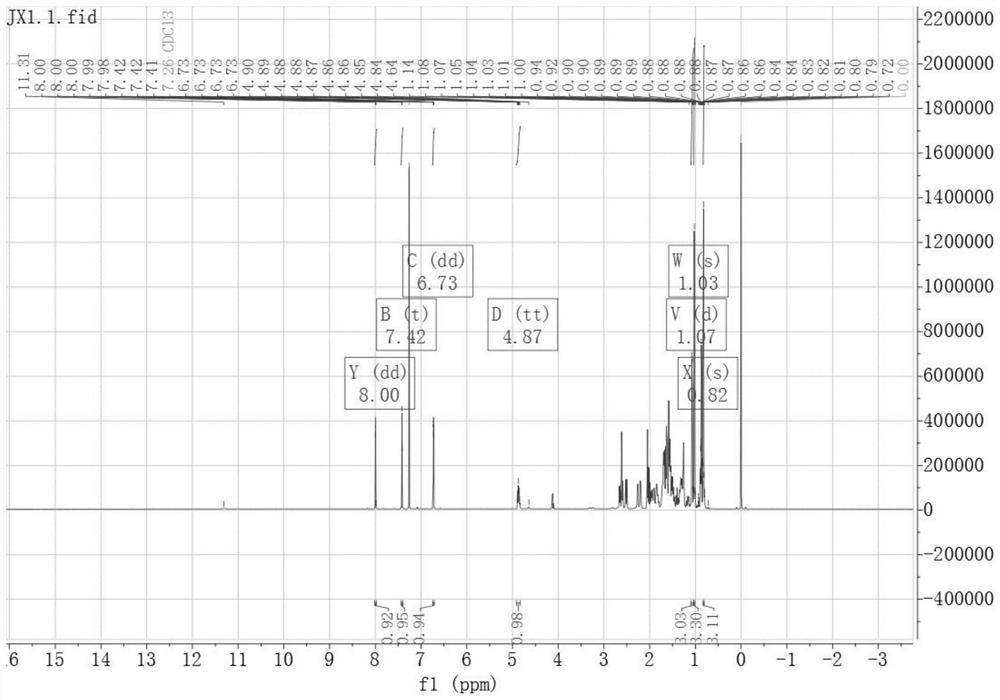
[0043] Embodiment one, peiminin B derivative A 13 C-NMR spectrum and 1 H-NMR spectrum, such as figure 1 and figure 2 as shown,
[0044]
[0045] Its preparation method is: dissolve 3-furancarboxylic acid (16.81mg, 0.15mmol) and DMAP (24.43mg, 0.2mmol) in dichloromethane, stir for 10min, then add peiminin (42.96mg, 0.1mmol) and EDCI (38.34mg, 0.2mmol), stirred and dissolved, and left at room temperature for 6h. After concentration under reduced pressure, silica gel thin-layer preparation plate was purified to obtain 47.816 mg of white powder with a yield of 80%. 1 H-NMR (400MHz, CDCl 3 )δppm: 8.00 (dd, J = 1.6, 0.8Hz, 1H, 3'-H), 7.42 (t, J = 1.7Hz, 1H, 5'-H), 6.73 (dd, J = 1.9, 0.8Hz, 1H,4'-H), 4.87(tt,J=11.4,4.8Hz,1H,3-H), 1.07(d,J=7.0Hz,3H,27-H), 1.03(s,3H,21- H), 0.82(s,3H,19-H). 13 C-NMR (151MHz, CDCl 3 )δppm: 210.49 (C-6), 162.61 (C-1'), 147.69 (C-4'), 143.67 (C-3'), 119.74 (C-2'), 109.84 (C-5'), 73.14(C-3), 70.91(C-20), 70.27(C-22), 62.32(C-26), 61.77(C-18),...
Embodiment 2
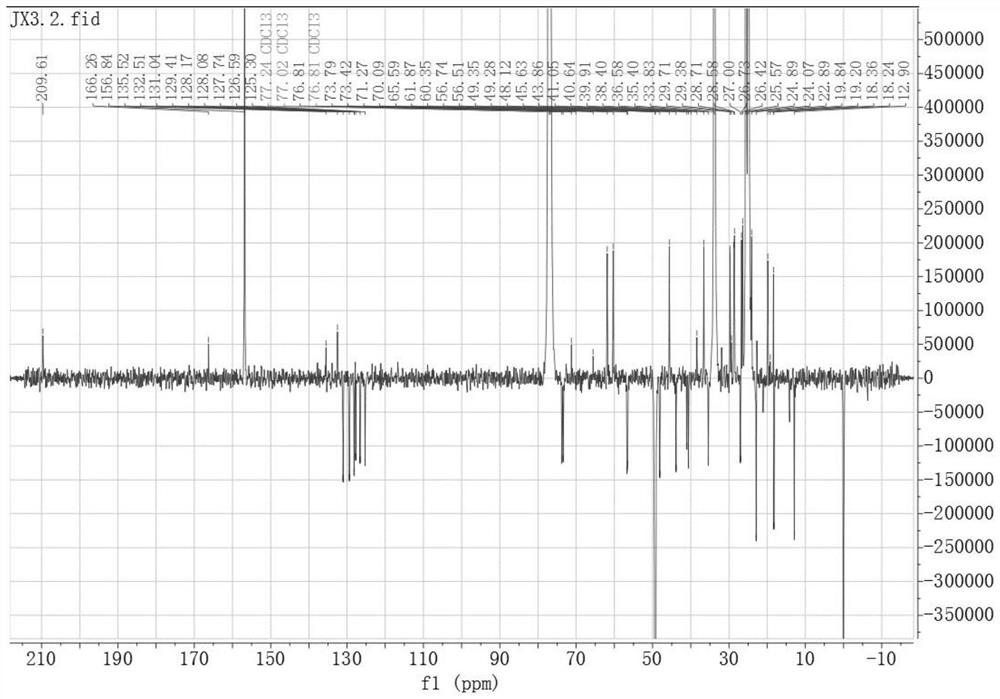
[0046]Embodiment two, peiminin B derivative B 13 C-NMR spectrum and 1 H-NMR spectrum, such as image 3 and Figure 4 as shown,
[0047]
[0048] Its preparation method is: dissolve 2-naphthoic acid (25.83mg, 0.15mmol) and DMAP (24.43mg, 0.27mmol) in dichloromethane, stir for 10min, then add peiminin (42.96mg, 0.1mmol) and EDCI (38.34mg, 0.2mmol), stirred and dissolved, and left at room temperature for 6h. After concentration under reduced pressure, silica gel thin-layer preparation plate was purified to obtain 53.74 mg of light yellow powder with a yield of 78.12%. . 1 H NMR (600MHz, CDCl 3 )δppm: 8.60(t, 1H, 3'-H), 8.05(dd, J=8.6, 1.7Hz, 1H, 9'-H), 7.97(m, 1H, 8'-H), 7.88(m, 1H,4'-H), 7.86(s,1H,7'-H), 7.59(m,1H,5'-H), 7.54(m,1H,6'-H), 4.99(1H,tt, 3-H), 1.10(s, 3H, 27-H), 0.98(s, 3H, 21-H), 0.88(m, 3H, 19-H). 13 C NMR (151MHz, CDCl 3 )δppm: 209.61 (C-6), 166.26 (C-1'), 135.52 (C-11'), 132.51 (C-10'), 131.04 (C-3'), 129.41 (C-4'), 128.17(C-6'), 128.08(C-8'), 127.8...
Embodiment 3
[0049] Embodiment three, peiminin B derivative C 13 C-NMR spectrum and 1 H-NMR spectrum, such as Figure 5 and Figure 6 as shown,
[0050]
[0051] Its preparation method is: dissolve 3-indoleacetic acid (26.27mg, 0.15mmol) and DMAP (24.43mg, 0.27mmol) in dichloromethane, stir for 10min, then add peiminin (42.96mg, 0.1mmol) and EDCI (38.34mg, 0.2mmol), stirred and dissolved, and left at room temperature for 6h. After concentration under reduced pressure, silica gel thin-layer preparation plate was purified to obtain 54.78 mg of light yellow powder with a yield of 78%. 1 H-NMR (300MHz, CDCl 3 )δppm: 7.62 (dd, J = 7.9, 1.2Hz, 1H, 8'-H), 7.35 (m, 1H, 5'-H), 7.20 (m, 1H, 4'-H), 7.17 (dd, J=11.2,1.8Hz,1H,6'-H), 7.13(dd,J=8.1,7.0Hz,1H,7'-H), 4.62(tt,J=11.4,4.8Hz,1H,3-H ), 1.09 (d, J=7.8Hz, 3H, 27-H), 1.04 (s, 3H, 21-H), 0.77 (s, 3H, 19-H). 13 C NMR (151MHz, CDCl 3 )δppm: 210.49(C-6), 171.49(C-1'), 136.11(C-10'), 127.27(C-9'), 122.98(C-4'), 122.16(C-6'), 119.61(C-7'), 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com