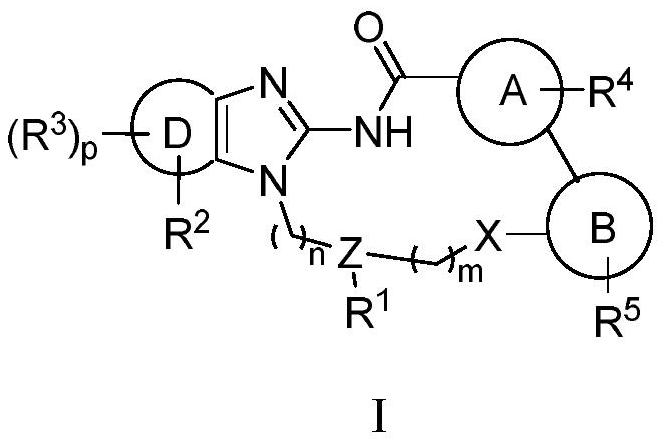
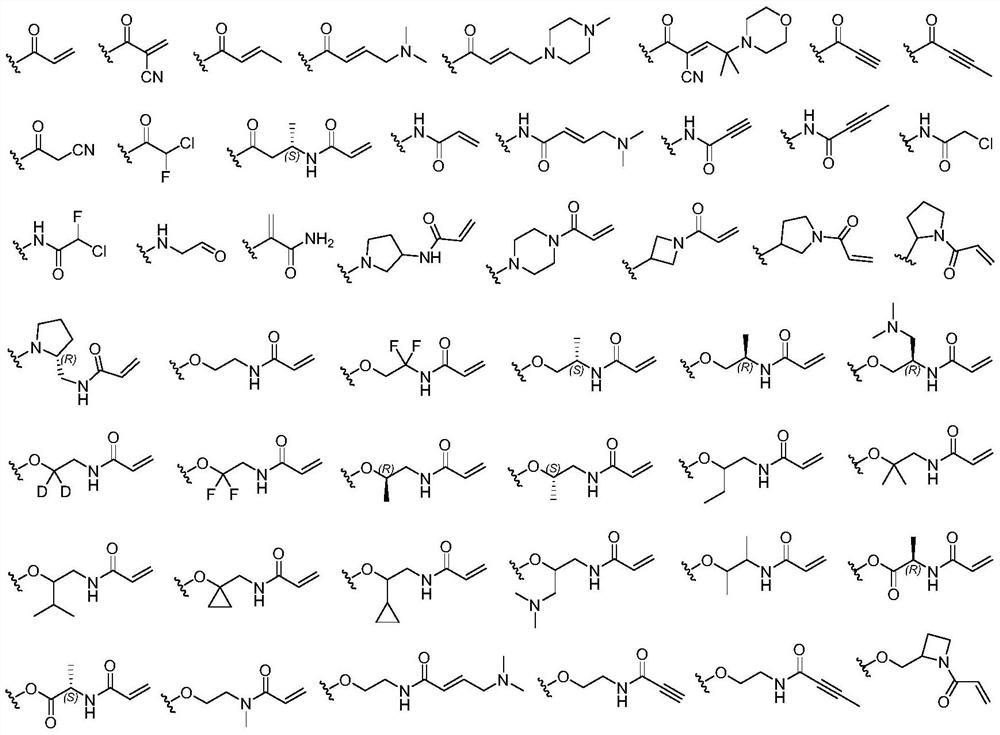
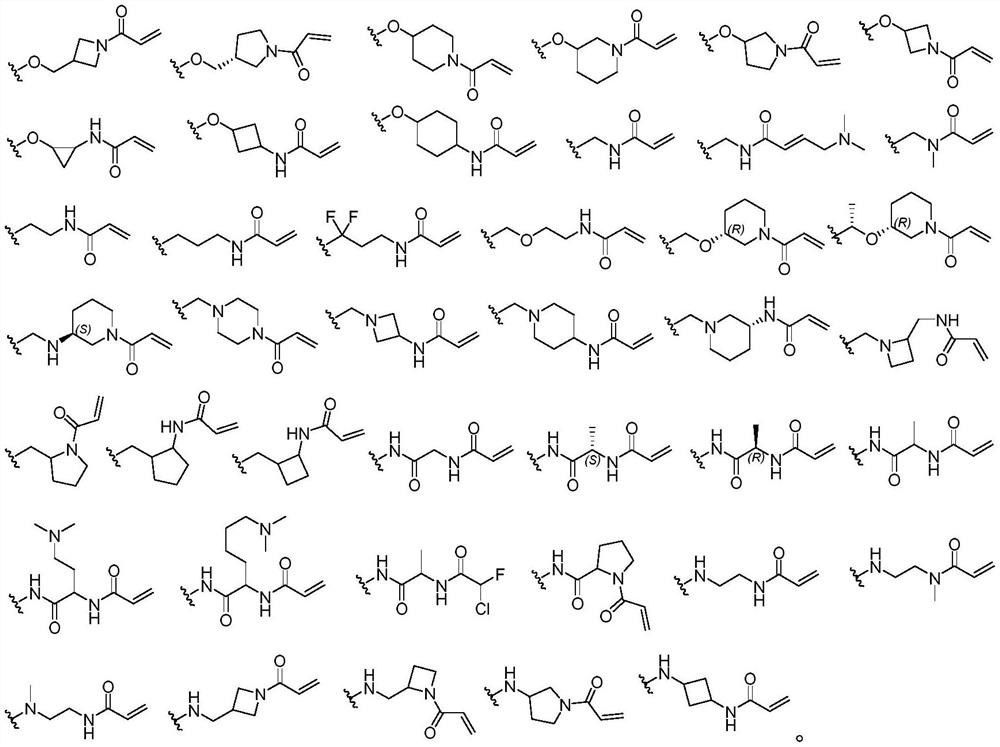
Macrocyclic compound as well as preparation method and application thereof
A technology of macrocyclic compounds and nitrogen oxides, applied in organic chemistry, drug combination, pharmaceutical formulations, etc., can solve the problems that non-covalent compounds cannot overcome drug resistance, poor selectivity of T790M mutants, inevitable C797S drug resistance mutations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0302] The synthesis of embodiment 1.(2-aminoethyl) (2-hydroxyethyl) tert-butyl carbamate
[0303]
[0304] The first step: 2,2,2-trifluoro-N-(2-((2-hydroxyethyl)amino)ethyl)acetamide
[0305]
[0306] At 0°C, add 2-(2-aminoethylamine)ethanol (10.0g, 96.0mmol), dry diethyl ether (30mL) and ethyl trifluoroacetate (13.6g, 96.0mmol) successively into a 100mL round bottom flask, The resulting mixture was stirred at room temperature for 2 h, a white precipitate formed. After filtration, the filter cake was washed twice with ether (20mL×2), and dried to obtain 2,2,2-trifluoro-N-(2-((2-hydroxyethyl)amino)ethyl)acetamide (14.6g, White solid), yield: 74.9%.
[0307] The second step: tert-butyl (2-hydroxyethyl)(2-(2,2,2-trifluoroacetylamino)ethyl)carbamate
[0308]
[0309] At room temperature, 2,2,2-trifluoro-N-(2-((2-hydroxyethyl)amino)ethyl)acetamide (13.4g, 0.066mol) was successively added to a dry 500mL round bottom flask, Tetrahydrofuran (200mL) and Boc 2 O (14.5g, 0...
Embodiment 2
[0315] The synthesis of embodiment 2.5-amino-4-methylpentan-1-alcohol
[0316]
[0317] The first step: tert-amyl (2,6-dimethylhept-5-en-1-yl)carbamate
[0318]
[0319] Dissolve 3,7-dimethyloct-6-enoic acid (3.5g, 20.56mmol) in toluene (300mL), heat up to 50°C, add triethylamine (2.5g, 24.67mmol), and then slowly add Diphenyl phosphate nitrogen (5.65g, 20.56mmol), heated to 70°C and stirred for 12h, added 2-methylbutan-2-ol (3.6g, 41.16mmol), heated to 110°C and stirred for 2h, then 2-Methylbutan-2-ol (3.6 g, 41.16 mmol) was added. The reaction mixture was stirred at 110 °C for 12 h. Concentrate under reduced pressure, add water (100 mL) to the obtained residue, extract with ethyl acetate (200 mL×3), combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure to obtain The residue was purified by silica gel column chromatography with eluent system petroleum ether / ethyl acetate=6...
Embodiment 3
[0326] Example 3. Synthesis of (R)-1-((tert-butoxycarbonyl)amino)propan-2-yl 4-toluenesulfonate
[0327]
[0328] The first step: (R)-(2-hydroxypropyl) tert-butyl carbamate
[0329]
[0330] At room temperature, (R)-1-aminopropan-2-ol (1.5g, 0.02mol) and DCM (20mL) were successively added to a 100mL round bottom flask, cooled to 0°C, and Boc 2 O (4.8g, 0.022mol) and triethylamine (4.0g, 0.04mol) were warmed up to room temperature and stirred for 2h. Add water (20 mL), extract with dichloromethane (20 mL×3), combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure. Removal system petroleum ether / ethyl acetate=5 / 1 was purified to obtain (R)-(2-hydroxypropyl)carbamate tert-butyl ester (2.4 g, white solid), yield: 68.6%.
[0331] The second step: (R)-1-((tert-butoxycarbonyl)amino)propan-2-yl 4-toluenesulfonate
[0332]
[0333] At room temperature, successively add tert-butyl (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com