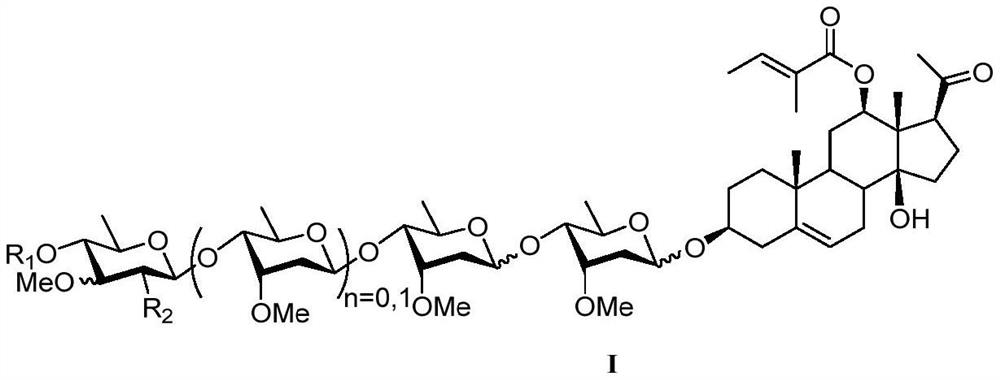
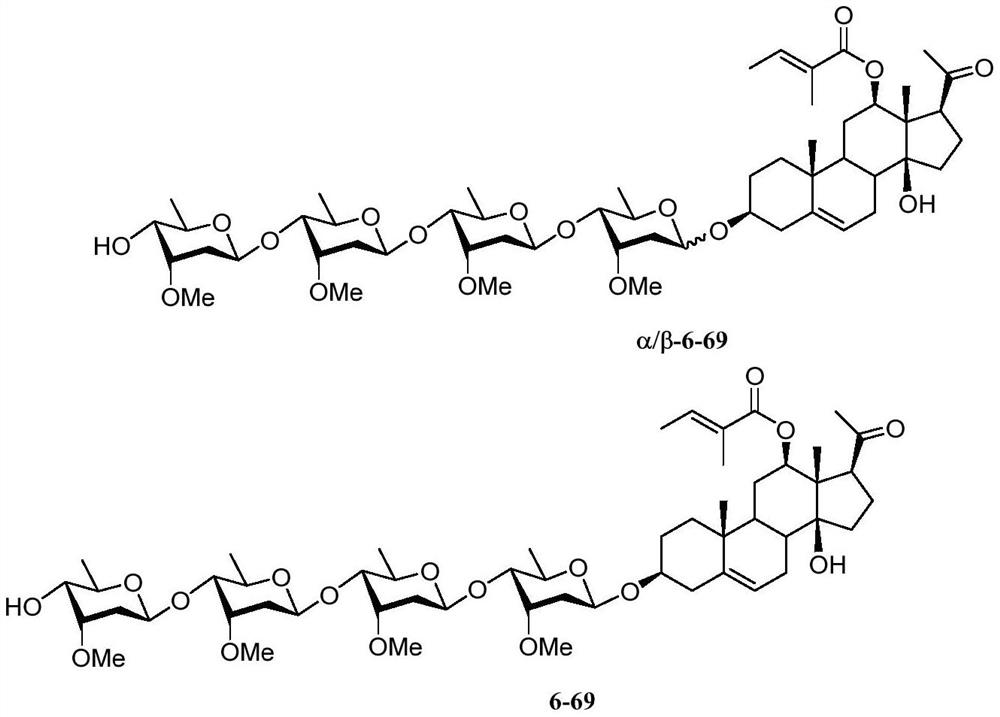
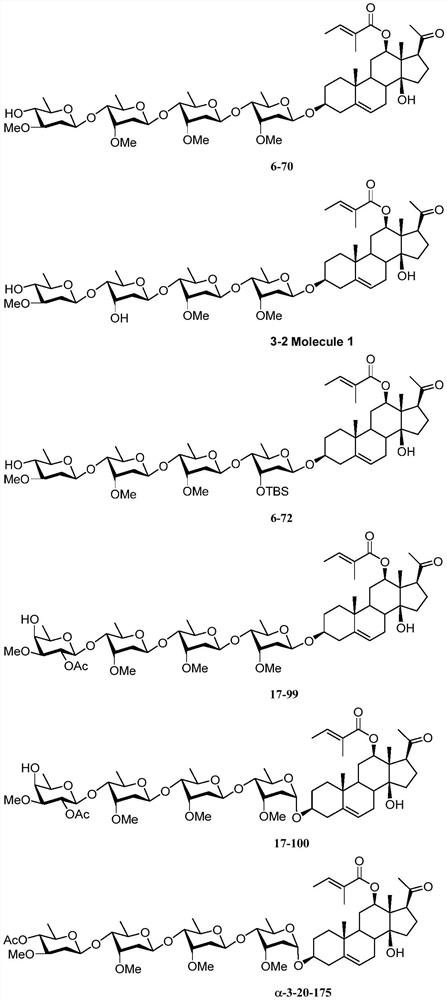
Intermediates of pregnane saponin P57 and derivatives thereof, preparation method and application of intermediates, and pregnane saponin P57 derivatives
A compound and selected technology, applied in the directions of steroids, chemical instruments and methods, glycoside steroids, etc., can solve the problems of troublesome separation and purification, lack of synthesis of P57, low yield of glycosylation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0069] The preparation method of compound P57
[0070] The steps of the compound P57 preparation method of the present invention are as follows:
[0071]
[0072] Step (1): Construction of the 12-position β hydroxyl group: References (Angew.Chem.Int.Ed.2009, 48, 7911-7914; J.Am.Chem.Soc.2015, 137, 13776-13779.), to Dehydroepiandrosterone (DHEA) is prepared as a raw material through three known reactions (3-hydroxyl protecting group protection, 17-position carbonyl to form enamine, long-range C-H bond activation to form 12-hydroxyl). Among them, R 4 from C 1 -C 6 Alkanoyl, benzoyl or para-substituted benzoyl, three (C 3 -C 9 Alkyl)silyl, three (C 9 -C 16 Aryl) silyl, allyl, benzyl or substituted benzyl on the benzene ring, or hydrogen; the para-substituent of the para-substituted benzoyl is methoxy, nitro, azide Base, halogen; substituted benzyl on the benzene ring refers to naphthylidene, p-methoxyphenyl, p-methylphenyl, p-nitrobenzyl, and p-halogen substituted benz...
Embodiment 1
[0106] The synthesis of embodiment 1 compound 2-4
[0107]
[0108] According to the method of literature (Angew.Chem.Int.Ed.2009,48,7911-7914; J.Am.Chem.Soc.2015,137,13776-13779.), using dehydroepiandrosterone as raw material, after 3 Compound 2-2 was prepared by one-step reaction. Compound 2-2: 1 H NMR (400MHz, CDCl 3)δ7.36-7.24(m,5H),5.37(brs,1H),4.56(s,2H),3.80(dd,J=11.3,4.7Hz,1H),3.31-3.23(m,1H),3.10 (s,1H),2.50-2.43(m,2H),2.31-2.26(m,1H),2.15-2.08(m,2H),2.04-1.94(m,2H),1.89-1.78(m,2H) ,1.68-1.40(m,5H),1.27-1.20(m,1H),1.13-1.00(m,2H),1.03(s,3H),0.95(s,3H); 13 C NMR (100MHz, CDCl 3 )δ141.2, 139.0, 128.4, 127.6, 127.5, 120.7, 78.3, 72.7, 70.0, 51.4, 49.6, 49.2, 39.1, 37.2, 37.2, 35.8, 30.6, 30.5, 28.3, 28.3, 21.7, 19.4, 8.1; 7ESIMS5[ M+Na] + .
[0109] Add Ph to the dry three-neck flask 3 Add THF (200mL) to PEtBr (28.5g, 76.8mmol) and t-BuOK (8.60g, 76.8mmol), and stir at room temperature for 40min to obtain a blood-red turbid liquid. Compound 2-2 (10.1g dissolv...
Embodiment 2
[0111] The synthesis of embodiment 2 compound 2-5
[0112]
[0113] Compound 2-4 (6.70g, 15.8mmol) was dissolved in dry DMF (150mL), imidazole (3.23g, 47.4mmol) and tert-butyldimethylsilyl chloride (3.57g, 23.7mmol) were added, and the reaction was stirred at room temperature 20h. TLC showed that the reaction was complete, diluted with ethyl acetate, washed successively with saturated aqueous sodium bicarbonate and saturated brine, dried over anhydrous sodium sulfate, concentrated, column chromatography (PE / EtOAc=12:1), and a white solid ( 8.2 g, 96%).
[0114] The above white solid (9.20 g, 17.1 mmol) was dissolved in dry CH 2 Cl 2 (5mL), add Dess-Martin oxidant (DMP) (18.1g, 42.7mmol) and NaHCO 3 (5.0g, 59.9mmol), stirred for 2h. TLC showed that the reaction was complete, diluted with EtOAc, followed by saturated Na 2 SO 3 The solution was washed with saturated aqueous sodium bicarbonate and saturated brine, dried over anhydrous sodium sulfate, concentrated, and su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com