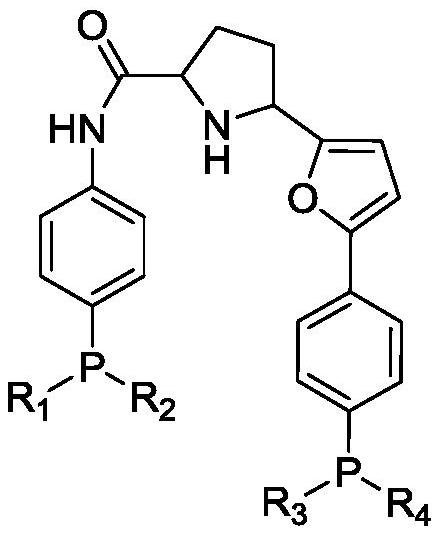
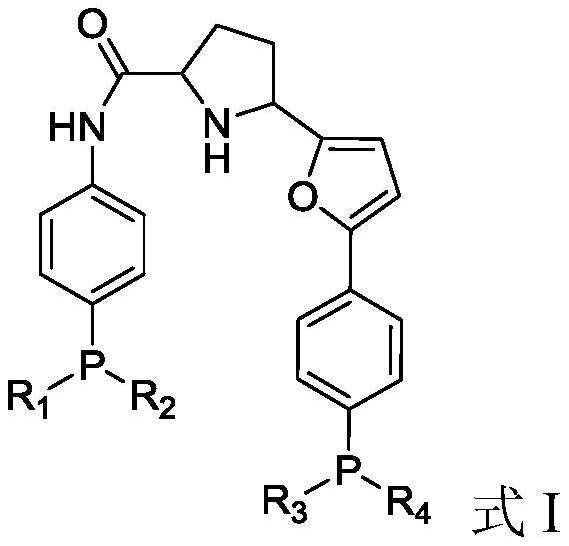
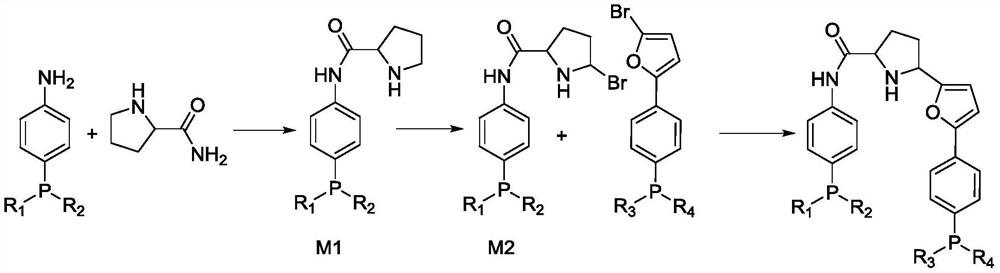
Bidentate phosphine ligand, hydroformylation catalyst and method for preparing linear dihydric alcohol from unsaturated fatty acid
A bidentate phosphine and catalyst technology, applied in the preparation of linear diols by catalyzing the hydroformylation of unsaturated fatty acids, in the field of bidentate phosphine ligands and hydroformylation catalysts, can solve the problem of low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] (1) Preparation of catalyst
[0053] Preparation of M2: 4-(diphenylphosphino)aniline (277.3g, 1mol), pyrrolidine-2-carboxamide (114.2g, 1.0mol), rhodium triphenylphosphine acetylacetonate carbonyl (2.46g, 0.005 mol) was dissolved in toluene, the temperature was raised to 90° C., and reacted for 1.0 hour. After cooling down to room temperature, NBS (213.6 g, 1.2 mol) was added, and reacted at room temperature for 0.5 hour to obtain M2 (430.7 g, 0.95 mol).
[0054] Elemental analysis: C: 60.96; H: 4.91; N: 6.13; P: 6.85; O: 3.56;
[0055] 1H NMR (500MHz, Chloroform-d) δ9.18(s,1H),7.59(ddd,2H),7.39–7.26(m,12H),4.73(dt,1H),4.45(t,1H),3.91( dt,1H),2.16–2.05(m,2H),1.86(ddt,2H).
[0056] Preparation of the ligand: M2 (430.7g, 0.95mol), (4-(5-bromofuran-2-yl)phenyl)diphenylphosphine (386.9g, 0.95mol), Mg powder (27.4g, 1.14 mol), tetramethylethylenediamine (132.5g, 1.14mol), FeCl 3 (7.7g, 0.047mol) was added into tetrahydrofuran and reacted under ice-cooling for 2.0 hours t...
Embodiment 2
[0062] (1) Preparation of catalyst
[0063] Preparation of M2: 4-(dithienylphosphino)aniline (289.4g, 1mol), pyrrolidine-2-carboxamide (171.2g, 1.5mol), rhodium triphenylphosphine acetylacetonate carbonyl (3.45g, 0.007 mol) was dissolved in toluene, the temperature was raised to 90°C, and reacted for 1.0 hour. After cooling down to room temperature, NBS (267.0 g, 1.5 mol) was added, and reacted at room temperature for 1.0 hour to obtain M2 (446.7 g, 0.96 mol).
[0064] Elemental analysis: C: 49.08; H: 3.95; N: 6.05; P: 6.68; O: 3.48; S: 13.79;
[0065] 1H NMR (500MHz, Chloroform-d) δ9.18(s,1H),7.63–7.55(m,2H),7.46(dd,2H),7.27–7.20(m,4H),7.09(ddd,2H), 4.73(dt,1H),4.45(s,1H),3.91(dt,1H),2.16–2.05(m,2H),1.91–1.81(m,2H).
[0066] Preparation of the ligand: M2 (446.7g, 0.96mol), (4-(5-bromofuran-2-yl)phenyl)dithienylphosphine (603.8g, 1.44mol), Mg powder (34.6g, 1.44 mol), tetramethylethylenediamine (132.5g, 1.14mol), FeCl 3 (12.5g, 0.05mol) was added into tetrahydrofuran, and ...
Embodiment 3
[0072] (1) Preparation of catalyst
[0073] Preparation of M2: 4-(diisopentylphosphino)aniline (265.4g, 1.0mol), pyrrolidine-2-carboxamide (137.0g, 1.2mol), rhodium triphenylphosphine acetylacetonate carbonyl (2.95g , 0.006mol) was dissolved in toluene, the temperature was raised to 110°C, and reacted for 1.5 hours. After cooling down to room temperature, NBS (231.3g, 1.3mol) was added, and reacted at room temperature for 1.0 hour to obtain M2 (423.7g, 0.96mol).
[0074] Elemental analysis: C: 57.18; H: 7.80; N: 6.35; P: 7.01; O: 3.61;
[0075] 1H NMR (500MHz, Chloroform-d) δ9.18(s,1H),7.50(tt,2H),7.34–7.28(m,2H),4.73(dt,1H),4.47–4.43(m,1H), 3.91(dt,1H),2.29(dtd,4H),2.16–2.05(m,2H),1.91–1.81(m,2H),1.54–1.35(m,6H),0.80–0.70(m,12H).
[0076] Ligand preparation: M2 (423.7g, 0.96mol), (4-(5-bromofuran-2-yl)phenyl) diisopentylphosphine (454.6g, 1.15mol), Mg powder (30.0g, 1.25mol), tetramethylethylenediamine (145.3g, 1.25mol), FeCl 3 (13.0 g, 0.08 mol) was added into tetrahydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com