Trelagliptin impurity compound and preparation method thereof
A technology of trexagliptin impurity and compound, which is applied in the field of drug synthesis, can solve the problems that the preparation method of trexagliptin impurity K has not been reported in literature, etc., and achieve the effects of high product purity, high reaction yield and short route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
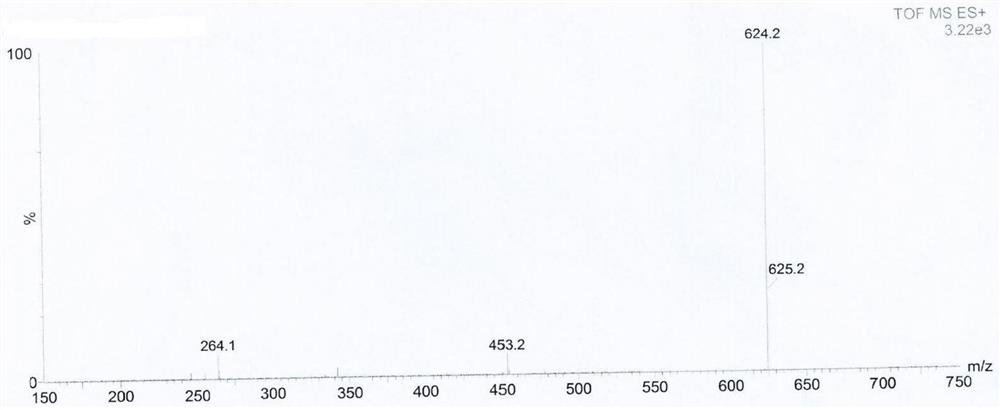
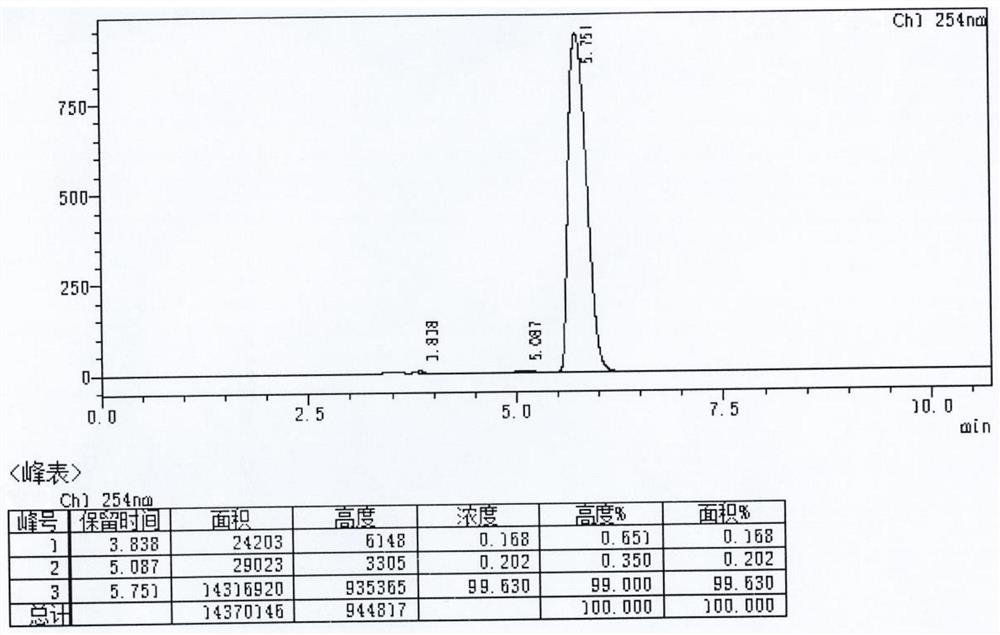
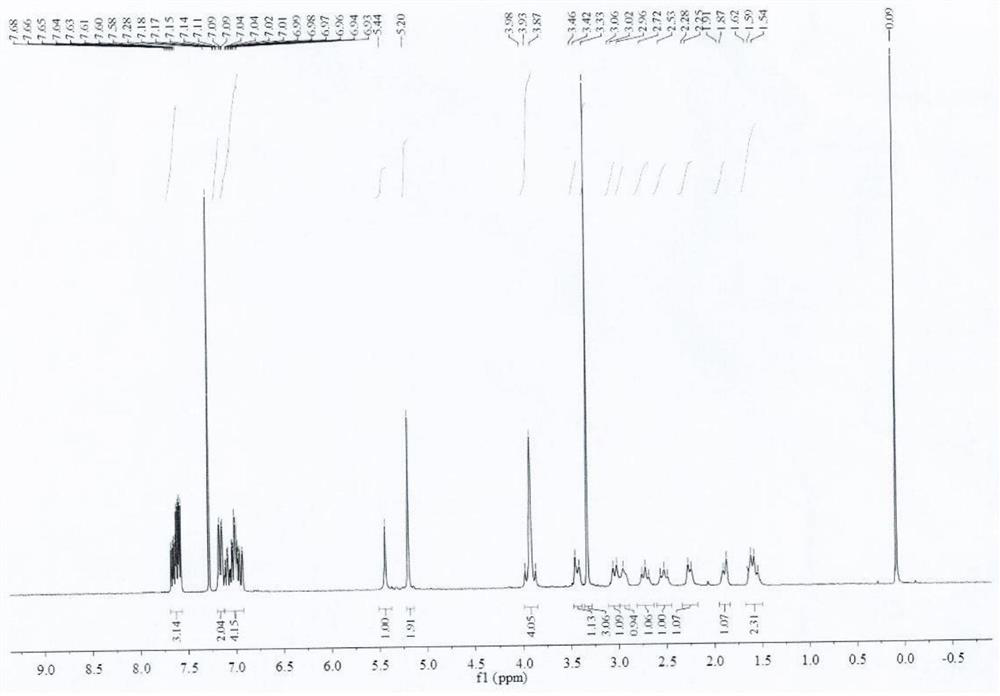
[0060] Add acetone (60ml) to a 250ml reaction flask, add compound I (R)-3-amino-1-Boc-piperidine (5.08g, 25.36mmol) and potassium carbonate (10.52g, 76.08mmol), and stir for 10min Afterwards, 2-cyano-5-fluorobenzyl bromide (11.94 g, 55.79 mmol) was added and stirring was continued at 20 °C for 16 h. Purified water (50ml) was added and the mixture was extracted with ethyl acetate (2 x 50ml). Dry the organic phase with anhydrous magnesium sulfate, filter and concentrate to obtain compound III (R)-3-(bis(2-cyano-5-fluorobenzyl))amino-1-Boc-piperidine with a yield of 95.5% , HPLC purity 99.91%.
Embodiment 2
[0062] Add acetone (60ml) in the reaction flask of 250ml, add compound I namely (R)-3-amino-1-Boc-piperidine (5.08g, 25.36mmol) and sodium bicarbonate (6.39g, 76.08mmol), stir After 10 min, 2-cyano-5-fluorobenzyl bromide (10.86 g, 50.72 mmol) was added and stirring was continued at 20 °C for 16 h. Purified water (50ml) was added and the mixture was extracted with ethyl acetate (2 x 50ml). Dry the organic phase with anhydrous magnesium sulfate, filter and concentrate to obtain compound III (R)-3-(bis(2-cyano-5-fluorobenzyl))amino-1-Boc-piperidine with a yield of 92.0% , HPLC purity 99.84%.
Embodiment 3
[0064] In the reaction bottle of 250ml, add acetone (60ml), add compound I namely (R)-3-amino-1-Boc-piperidine (5.08g, 25.36mmol) and triethylamine (7.70g, 76.08mmol), stir After 10 min, 2-cyano-5-fluorobenzyl bromide (16.28 g, 76.08 mmol) was added and stirring was continued at 20 °C for 16 h. Purified water (50ml) was added and the mixture was extracted with ethyl acetate (2 x 50ml). Dry the organic phase with anhydrous magnesium sulfate, filter and concentrate to obtain compound III (R)-3-(bis(2-cyano-5-fluorobenzyl))amino-1-Boc-piperidine with a yield of 87.8% , HPLC purity 99.63%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com