Application of reagent for detecting expression level of RIG-I-Shart in preparation of products for tumor diagnosis and/or prognosis
An expression level, tumor diagnosis technology, applied in expression level reagents, application fields in products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
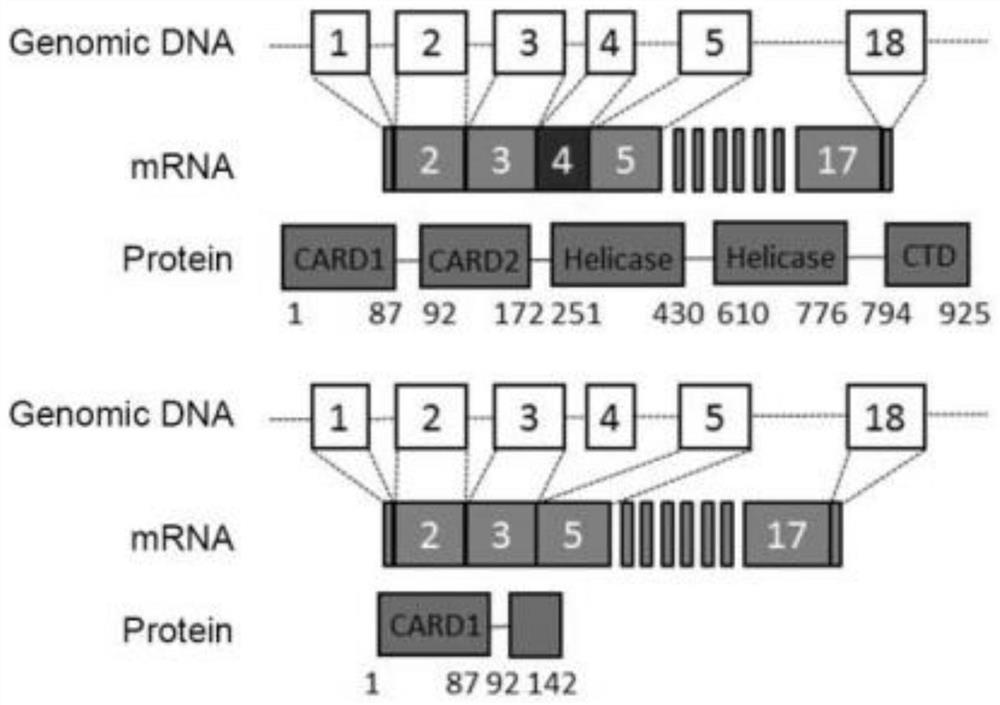
[0055] Example 1 RIG-I-Short is an alternative cut form of RIG-I induced by interferon
[0056] (1) Identification of RIG-I-Short protein sequence and mRNA sequence
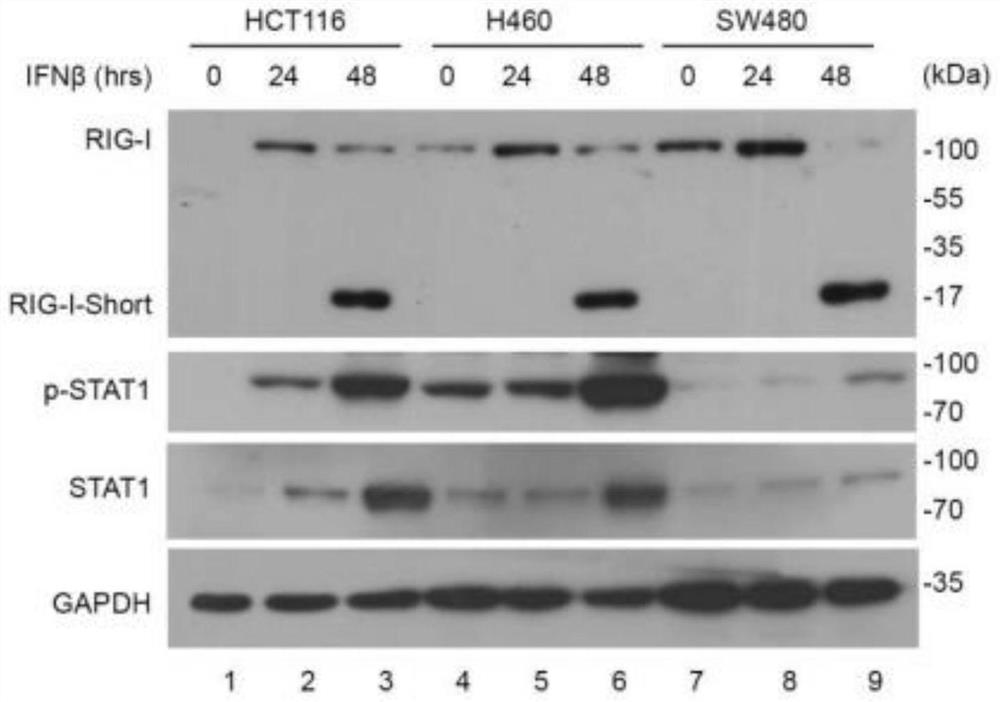
[0057] RIG-I is a typical interferon-stimulated gene, and its expression will be up-regulated under virus infection and interferon-stimulated conditions. This result is verified by the present invention through Western blotting and quantitative real-time PCR experiments. The results of gel electrophoresis experiments showed that: in human cells, the RIG-I N-terminal specific antibody can recognize a protein located at a size of 17kDa ( figure 1 ).
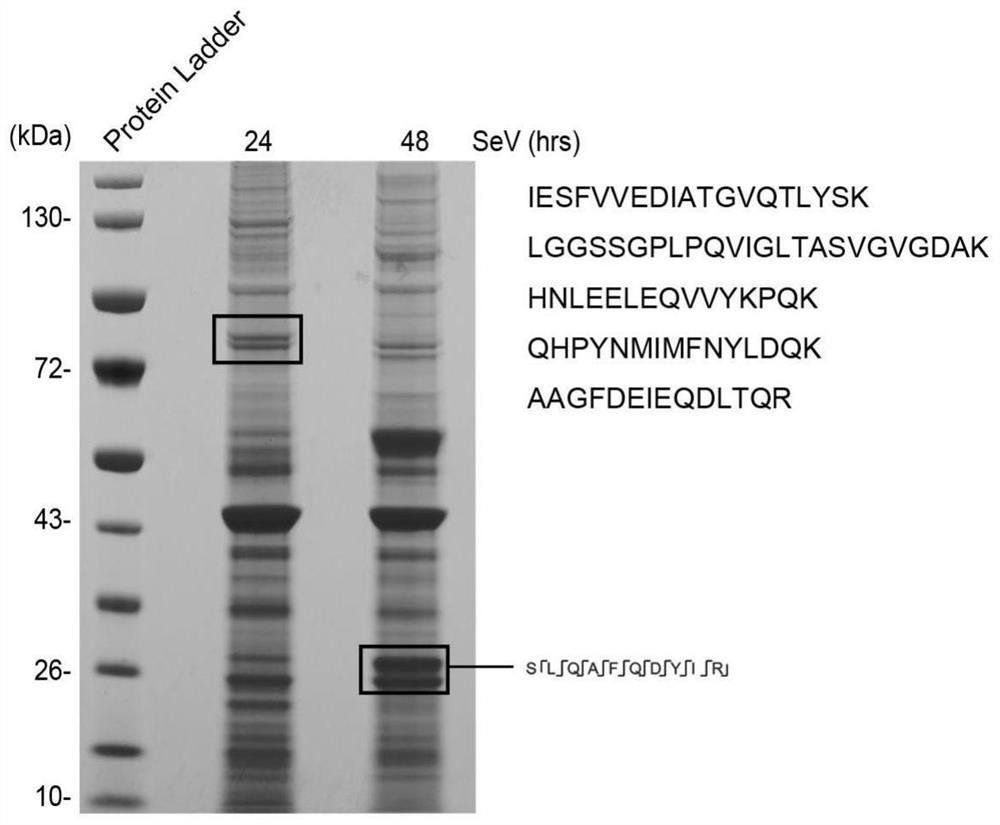
[0058] In order to further confirm that the protein corresponding to the RIG-I N-terminal antibody recognition band is the RIG-I subtype, the present invention infected H460 cells with Sendai virus, and used the RIG-I N-terminal antibody to enrich the protein at 24 hours and 48 hours, and passed Protein gel electrophoresis separated proteins of different sizes, and ...
Embodiment 2
[0067] Example 2 Physiological and pathological functions of RIG-I-Short
[0068] (1) RIG-I-Short inhibits the activity of transcription factor STAT1
[0069] In order to explore the physiological function of RIG-I-Short, the present invention uses the method of FLAG pμlldown combined with mass spectrometry to identify proteins interacting with RIG-I-Short, and finds that the transcription factor STAT1, which plays an important role in the interferon pathway, can interact with RIG-I -Short combination ( Figure 15 ). Subsequently, the present invention verified the interaction between RIG-I-Short and STAT family members, and co-immunoprecipitation experiments revealed that RIG-I-Short interacted with STAT1 and STAT2 ( Figure 16 ).
[0070]When the interferon binds to the interferon receptor, it recruits downstream kinases to phosphorylate STAT1, and the phosphorylated STAT1 forms a dimer, and then enters the nucleus to exert its transcription factor activity. In order to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com