Chimeric phenylalanine translation system introduced with non-natural amino acid and construction method of chimeric phenylalanine translation system
An unnatural amino acid, phenylalanine technology, applied in the field of molecular biology, can solve the problems of signal-to-noise ratio improvement, unreachable insertion efficiency of unnatural amino acids, and obstacles to the widespread application of chimeric phenylalanine translation systems, reaching The effect of high signal-to-noise ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
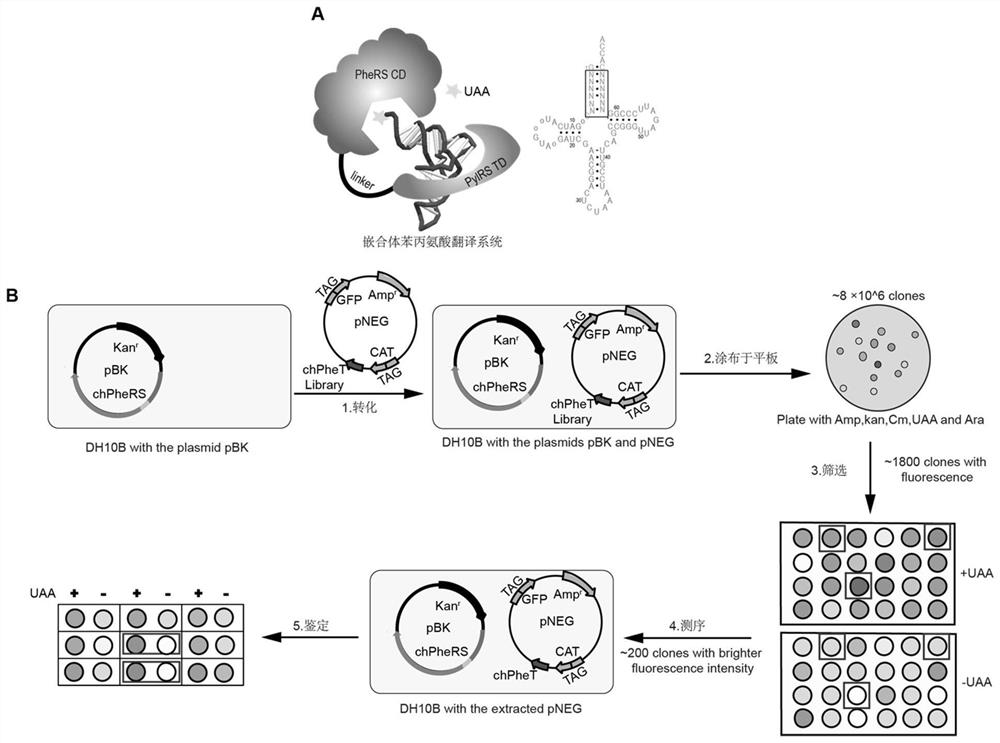
[0088] Example 1. Library construction of chimeric phenylalanine-tRNA
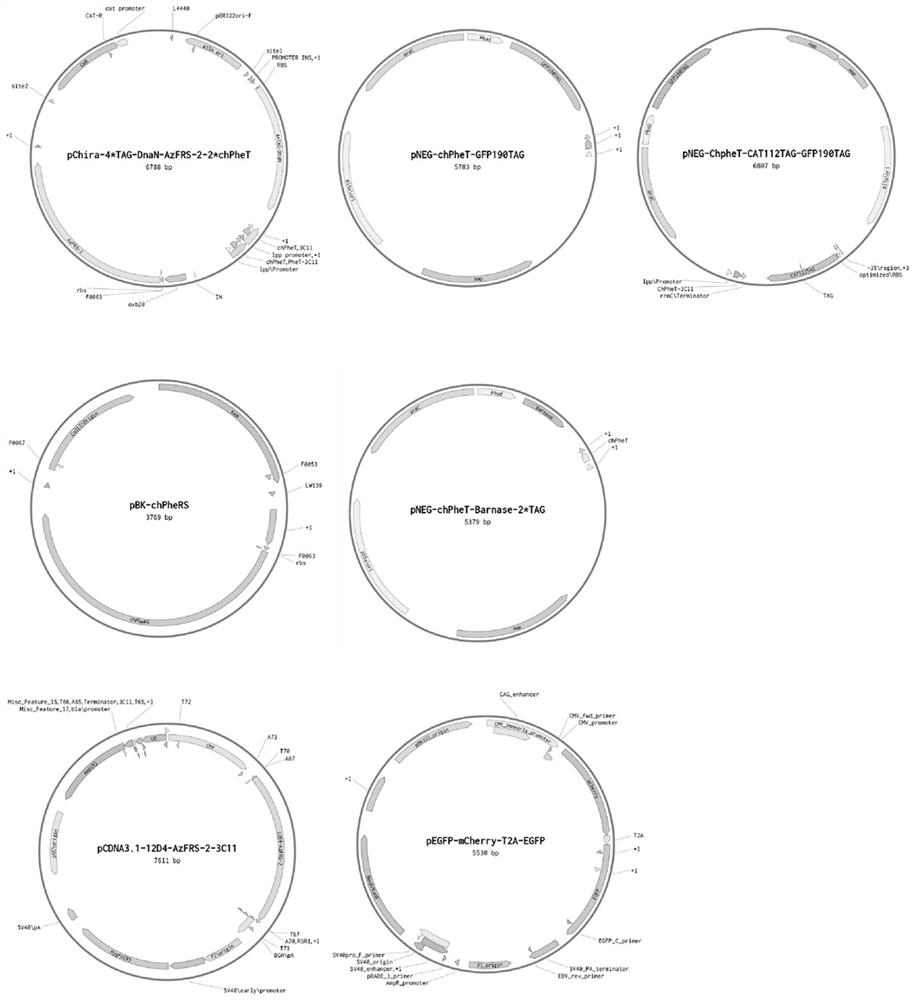
[0089] (1) The nucleotide sequence of the wild-type phenylalanine-tRNA is shown in SEQ ID NO: 1; the 2nd to 7th base pairs of the selected chimera phenylalanine-tRNA acceptor arm region, with pNEG-chPheT-GFP190TAG (see for details figure 1 , nucleotide sequence referring to SEQ ID NO: 45) as a template, with the primer PheT-Lib-AU-F / R (ie SEQ ID NO: 17 and SEQ ID NO: 18 shown in Table 1) saturation mutagenesis to produce approximately 1.7x10 7 The mutant library was cloned into pNEG-CAT-112TAG-GFP-190TAG-tRNA vector by Gibson assembly.
[0090] (2) Transform pBK-chPheRS-1 into Escherichia coli DH10B to prepare electroporation competent cells.
[0091] (3) Electrotransform the mutant library prepared in (1) into the electroporation-competent cells in (2), add 1mMAzF and incubate at 37°C for 3h, and then spread the bacterial solution on the cells containing 50μg / ml Kanamyces 100 μg / ml ampicillin, 10 μg / ...
Embodiment 2
[0094] Example 2. Screening chimeric phenylalanine-tRNA mutants by GFP fluorescence reporter assay
[0095] (1) Pick the single clone with fluorescent signal in Example 1 and culture overnight.
[0096] (2) Inoculate the bacterial solution in (1) according to the ratio of 1:100, and cultivate to OD at 37°C 600 When =0.6-0.8, add 0.2% arabinose to induce expression, and at the same time take 1 ml of bacterial liquid and add 1 mM AzF, and express at 30° C. for 20 h.
[0097] (3) After centrifuging 750 μl of the bacterial solution in (2), add 150 μl of 1×Bugbuster (Millipore, Lot: 3492682) and place it at 25°C for 30 min, then centrifuge, take 100 μl of the supernatant to a 96-well plate, and at the same time Take 100 μl (2) of the bacterial solution, and measure the GFP fluorescence signal intensity and OD by a microplate reader 600 , to calculate the mutant recognition efficiency of unnatural amino acids.
[0098] (4) Sequence the chimera phenylalanine-tRNA mutant that signi...
Embodiment 3
[0102] Example 3. Directed evolution of the catalytic domain of chimeric phenylalanyl-tRNA synthetases
[0103] (1) Using the C-terminal catalytic domain sequence (SEQ ID NO: 8) of the chimeric phenylalanyl-tRNA synthetase (chPheRS) as a template, perform error-prone PCR amplification to obtain the C-terminal catalytic domain mutation of chPheRS Libraries, mutant libraries were cloned into the pBK vector by Gibson assembly.
[0104] (2) Combine pNEG-chPheT-CAT112TAG-GFP190TAG (see figure 1 , the nucleotide sequence is SEQ ID NO:47) The plasmid was transformed into Escherichia coli DH10B to prepare electroporation competent cells.
[0105] (3) Electrotransforming the mutant library of the chimeric phenylalanyl-tRNA synthetase in (1) into the electroporation competent cells in (2), and culturing to obtain a single clone.
[0106] (4) Identify mutants that significantly improve the efficiency of the chimera phenylalanine translation system through GFP fluorescent signal report...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com