Method for crystallizing magnesium sulfate
A technology of magnesium sulfate and magnesium sulfate mother liquor, which is applied in the direction of magnesium sulfate, solution crystallization, heat exchange cooling crystallization, etc., can solve the problems of low energy consumption and low eutectic point temperature, achieve low energy consumption, increase eutectic temperature, The effect of improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
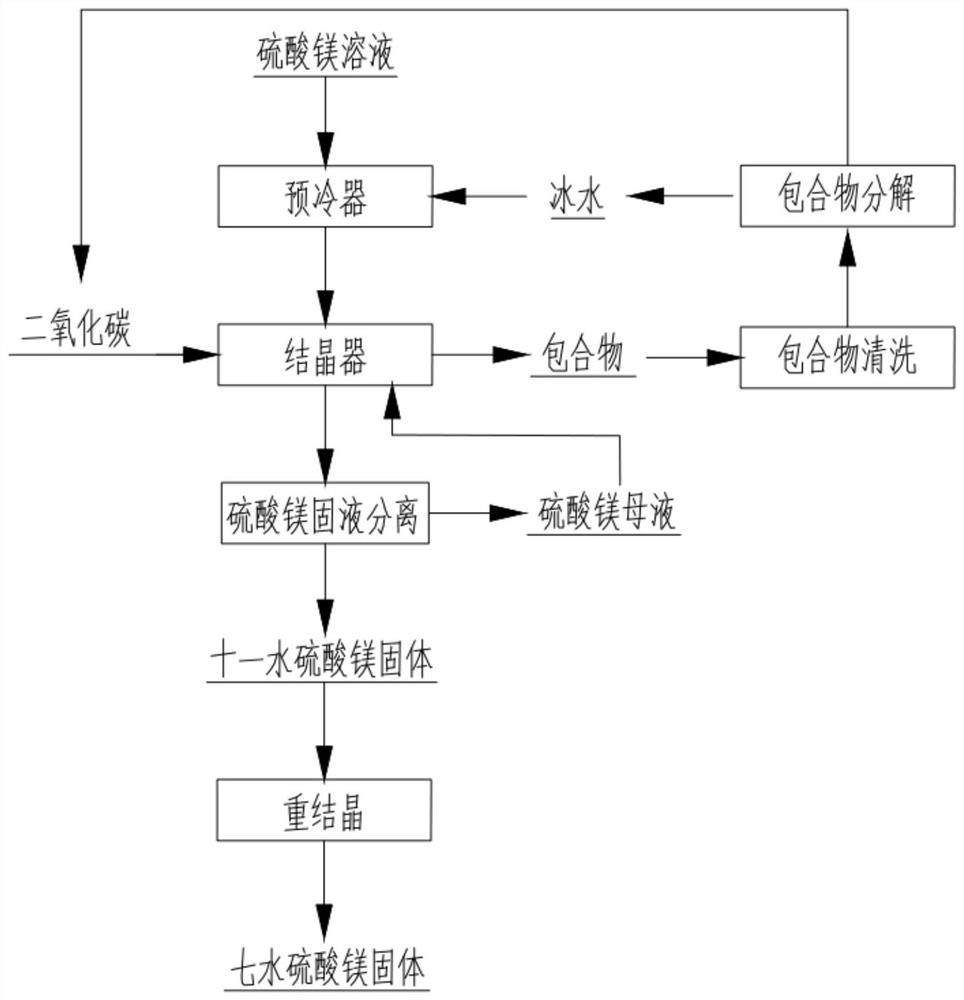
[0027] Prepare 5 L of magnesium sulfate solution with magnesium sulfate content of 17.3%. Ice water is used as the cooling medium to pre-cool 5L magnesium sulfate solution to 5°C through the shell and tube heat exchanger. The pre-cooled magnesium sulfate solution is pumped into the crystallizer, and the crystallizer is stirred to 250rpm. Inside the crystallizer, stop feeding carbon dioxide gas after the pressure shows 4.0Mpa, the ethylene glycol refrigerant continues to cool the magnesium sulfate solution through the jacket of the crystallizer, and stop precooling when the internal temperature of the crystallizer drops to 6.5°C, at this time CO 2 and feed valve closed. The pressure and temperature were maintained for an additional 1.0 hour, and crystallization was observed. The upper layer of the crystallizer forms a clathrate of carbon dioxide clathrate, and when the pressure is slightly lowered, CO is added 2 , otherwise it will lead to the decomposition of some carbon dio...
Embodiment 2
[0029] Prepare 5 L of magnesium sulfate solution with magnesium sulfate content of 17.3%. Ice water is used as the cooling medium to pre-cool 5L magnesium sulfate solution to 5°C through the shell and tube heat exchanger. The pre-cooled magnesium sulfate solution is pumped into the crystallizer, and the crystallizer is stirred to 250rpm. Inside the crystallizer, stop feeding carbon dioxide gas after the pressure shows 3.0Mpa. The ethylene glycol refrigerant continues to cool the magnesium sulfate solution through the jacket of the crystallizer. When the internal temperature of the crystallizer drops to 5.0°C, stop precooling. At this time, CO 2 and feed valve closed. The pressure and temperature were maintained for an additional 1.0 hour, and crystallization was observed. The upper layer of the crystallizer forms a clathrate of carbon dioxide clathrate, and when the pressure is slightly lowered, CO is added 2 , otherwise it will lead to the decomposition of some carbon dioxi...
Embodiment 3
[0031] Prepare 5 L of magnesium sulfate solution with magnesium sulfate content of 17.3%. Ice water is used as the cooling medium to pre-cool 5L magnesium sulfate solution to 5°C through the shell and tube heat exchanger. The pre-cooled magnesium sulfate solution is pumped into the crystallizer, and the crystallizer is stirred to 250rpm. Inside the crystallizer, stop feeding carbon dioxide gas after the pressure shows 2.0Mpa. The ethylene glycol refrigerant continues to cool the magnesium sulfate solution through the jacket of the crystallizer. When the internal temperature of the crystallizer drops to 1.9°C, stop precooling. At this time, CO 2 and feed valve closed. The pressure and temperature were maintained for an additional 1.0 hour, and crystallization was observed. The upper layer of the crystallizer forms a clathrate of carbon dioxide clathrate, and when the pressure is slightly lowered, CO is added 2 , otherwise it will lead to the decomposition of some carbon dioxi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com