Anti-coronavirus bispecific neutralizing antibody and application
A bispecific antibody and coronavirus technology, applied in antiviral agents, antiviral immunoglobulins, applications, etc., can solve problems such as immune escape, error, drug failure or reduced neutralization ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
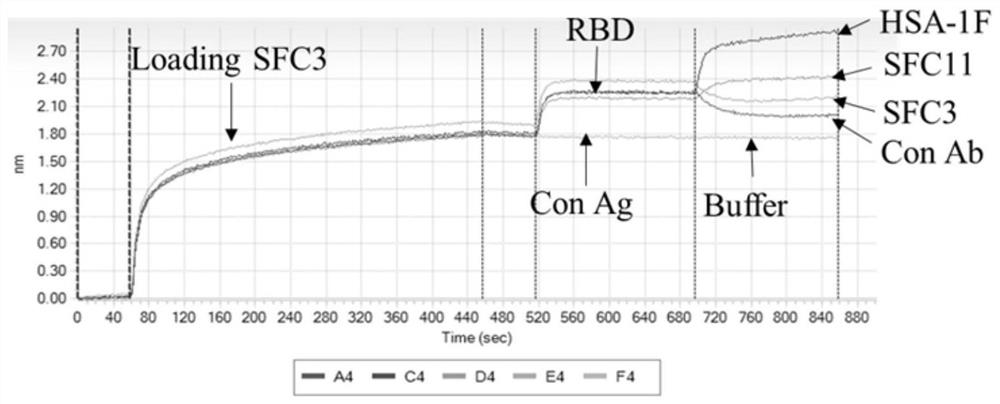
[0111] Example 1. Exploring the binding sites of parental antibodies SFC3 and HSA-1F to wild-type SARS-CoV-2 RBD
[0112] By analyzing the anti-COVID-19 virus monoclonal antibodies obtained in the previous work, the present inventor unexpectedly found that: the two murine monoclonal antibodies targeting the RBD region of the SARS-CoV-2 S protein obtained in the previous work can be Lower concentrations completely blocked the virus from infecting host cells, and showed a certain synergistic effect. In addition, the binding epitopes of the two monoclonal antibodies were identified by ForteBio based on BLI technology, and the epitopes of the two monoclonal antibodies binding to RBD were found. bits are different. On this basis, two humanized monoclonal antibodies, namely SFC3 and HSA-1F, were used to construct bispecific antibodies, and an IgG-like bispecific antibody BI31 was constructed. The bispecific antibody BI31 is obtained by modifying the parental antibodies SFC3 and HSA...
Embodiment 2
[0116] Example 2, Preparation of anti-new coronavirus bispecific antibody
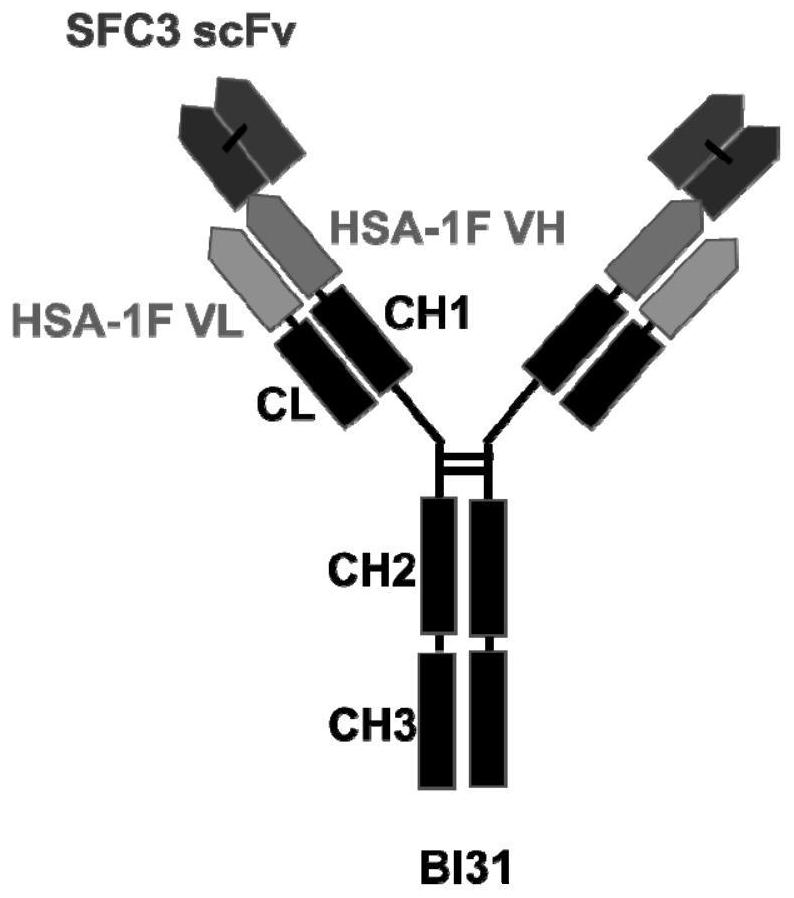
[0117] 1. The structure of the bispecific antibody BI31
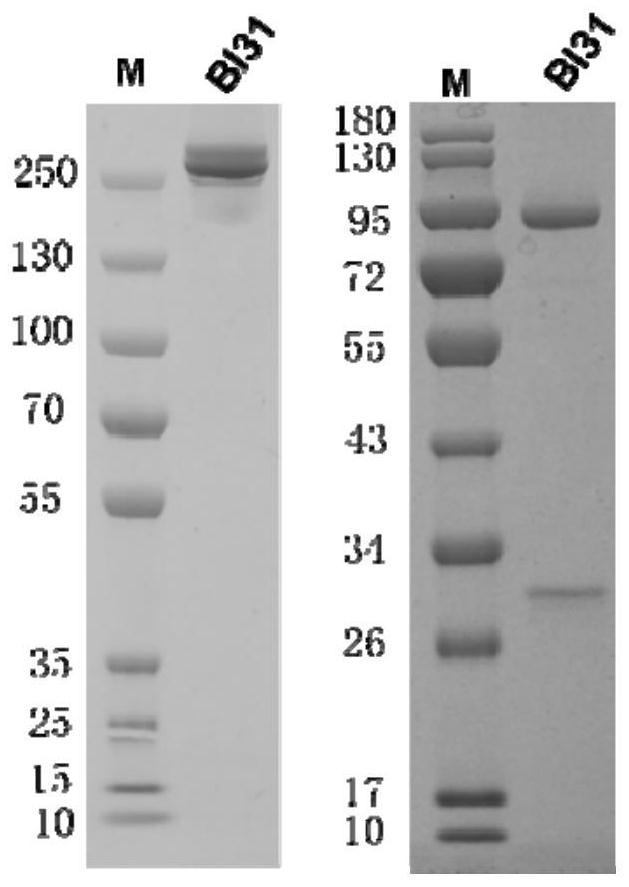
[0118] The IgG-like bispecific neutralizing antibody was prepared by using the humanized monoclonal antibody SFC3 and HSA-1F, named BI31 (the constant region of the heavy chain is IgG1, and the light chain is Kappa). The bispecific antibody BI31 is a scFv fused with SFC3 at the N-terminus of the humanized antibody HSA-1F (ie, the N-terminus of the heavy chain variable region of HSA-1F) (ie, the single-chain antibody SFC3 scFv). The single-chain antibody SFC3 scFv uses an oligonucleotide linker to connect the heavy chain variable region (VH) and light chain variable region (VL) of the humanized antibody SFC3 to form a single polypeptide chain . The constructed bispecific antibody BI31 contains the single-chain antibody SFC3scFv and the complete humanized antibody HSA-1F structure, which has a highly stable symmetrical structure (the structure o...
Embodiment 3、BI31
[0170] Example 3, Detection of specific binding ability of BI31 antibody and parental antibody
[0171] 1. Elisa detection antibody affinity to SARS-CoV-2 RBD protein
[0172] 1. Dilute SARS-CoV-2 RBD recombinant protein (Shenzhou, 40592-V08H5) with carbonate coating buffer (pH 9.6) to 2ng / ul, and add it to the microtiter plate (Corning, 9018), each experimental well was set up with 3 replicate wells, and coated overnight at 4°C.
[0173] 2. On the next day, wash the overnight coated ELISA plate with PBST 6 times, add PBS blocking solution containing 2% (mass percentage) skimmed milk powder, and incubate at 37°C for 2h.
[0174] 3. After blocking, discard the blocking solution, add 100 μL of 2-fold diluted BI31 antibody solution (initial concentration: 50 nM) to each well, set up a total of 11 gradients, incubate at 37°C for 90 minutes, and then wash the plate 6 times with PBST.
[0175] 4. After completing the above steps, take the ELISA plate, add 100 μL of 1:4000 dilution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com