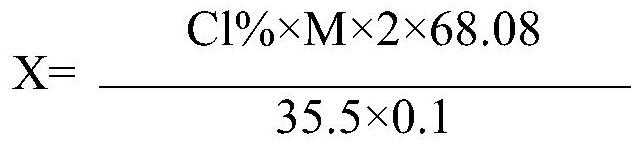Method for controlling synthesis end point of carbonyl diimidazole
A carbonyldiimidazole technology and a control method are applied in the control field of the synthetic end point of high-quality carbonyldiimidazole, and can solve the problems of high imidazole content, low yield and content, easy moisture absorption and dissolution of products, etc. The effect of simple process and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
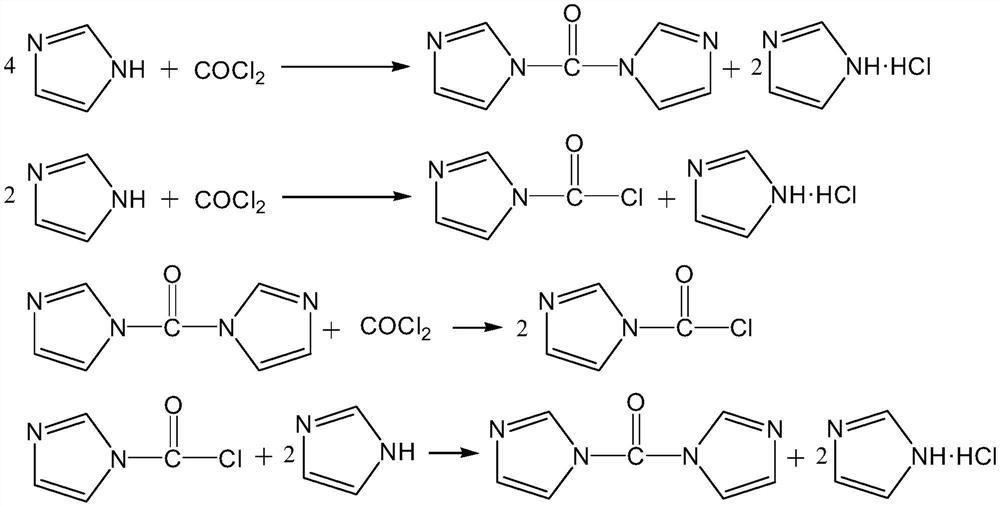
[0047] A method for controlling the synthetic end point of carbonyldiimidazole of the present invention, comprising the following steps:
[0048] in N 2 Under protection, 41.3g (0.6mol, 99%) of imidazole and 400g of tetrahydrofuran were put into a 1000mL four-neck flask with a condenser, and the stirring was started. The reaction temperature was controlled at 10° C., and 118 g of a THF solution containing phosgene was added dropwise within 1 hour. The THF solution containing phosgene contained 18.0 g (0.1818 mol) of phosgene. After the addition of phosgene is completed, continue to stir and keep warm for 30 minutes, turn off the stirring and let stand for 10 minutes, take the supernatant of the reaction solution and quantitatively measure the chloride ion content to be 0.018%. According to the chloride ion content, 3.86 g of a tetrahydrofuran solution of imidazole with a mass fraction of 10% was quantitatively added dropwise while stirring. After the dropwise addition is com...
Embodiment 2
[0050] A method for controlling the synthetic end point of carbonyldiimidazole of the present invention, comprising the following steps:
[0051] in N 2 Under protection, 41.3g (0.6mol, 99%) of imidazole and 400g of tetrahydrofuran were put into a 1000mL four-neck flask with a condenser, and the stirring was started. The reaction temperature was controlled at 10° C., and 119.4 g of a tetrahydrofuran solution of phosgene containing 19.3 g (0.1949 mol) of phosgene was added dropwise within 1 hour. After the addition of phosgene was completed, after 30 minutes of heat preservation, the stirring was turned off and allowed to stand for 10 minutes, and the supernatant of the reaction solution was taken to quantitatively measure the chloride ion content to be 0.031%. According to the chloride ion content, 6.67 g of 10% imidazole in tetrahydrofuran solution was quantitatively added during stirring. After the dropwise addition was completed, the chloride ions in the reaction liquid c...
Embodiment 3
[0053] A method for controlling the synthetic end point of carbonyldiimidazole of the present invention, comprising the following steps:
[0054] in N 2 Under protection, 41.3g (0.6mol, 99%) of imidazole and 400g of tetrahydrofuran were put into a 1000mL four-neck flask with a condenser, and the stirring was started. The reaction temperature was controlled at 10° C., and 98 g of a phosgene-tetrahydrofuran solution containing 19.1 g (0.1929 mol) of phosgene was added dropwise within 1 hour. After the addition of phosgene was completed, after 30 minutes of heat preservation, the stirring was turned off and allowed to stand for 10 minutes, and the supernatant of the reaction solution was taken to quantitatively measure the chloride ion content to be 0.034%. According to the chloride ion content, 7.03 g of a tetrahydrofuran solution of 10 wt % imidazole was quantitatively added during stirring. After the dropwise addition was completed, the chloride ions in the reaction liquid c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com