Acyclovir tricyclic nucleoside derivative as well as synthesis method and application thereof
A technology of nucleoside derivatives and synthesis methods, which is applied in the field of acyclovir tricyclic nucleoside derivatives and their synthesis, can solve the problems of patients prone to drug resistance and large toxic and side effects, and achieve simple operation and high yield. High efficiency and good chemoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
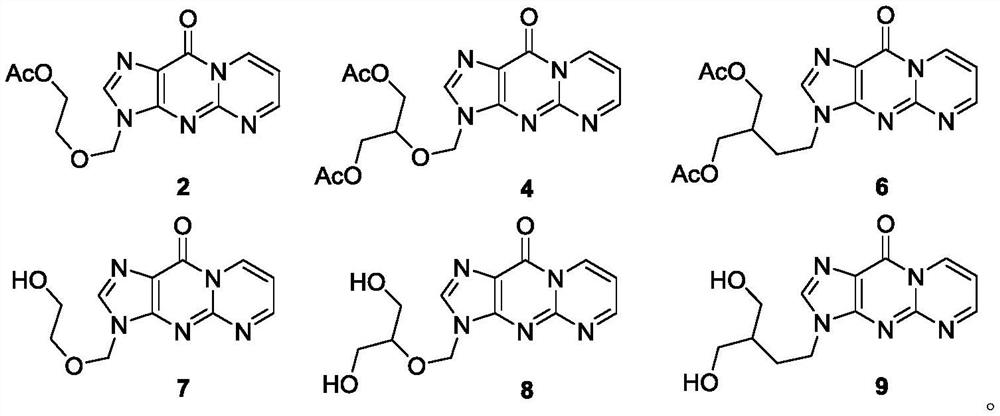
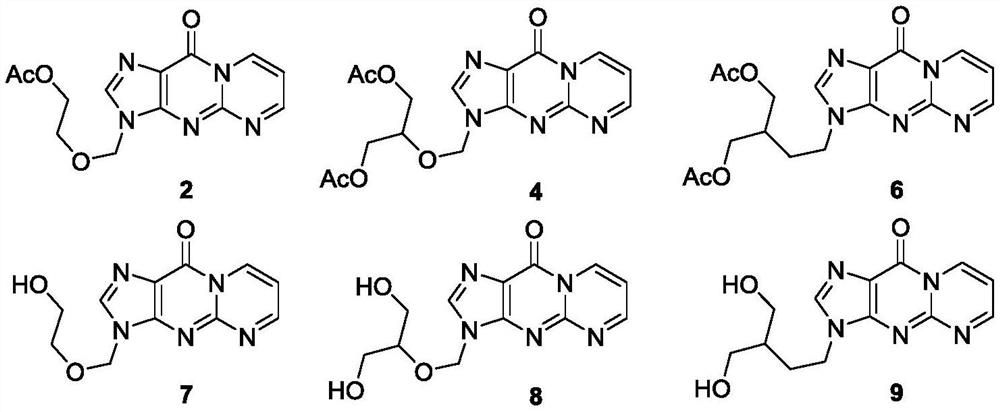
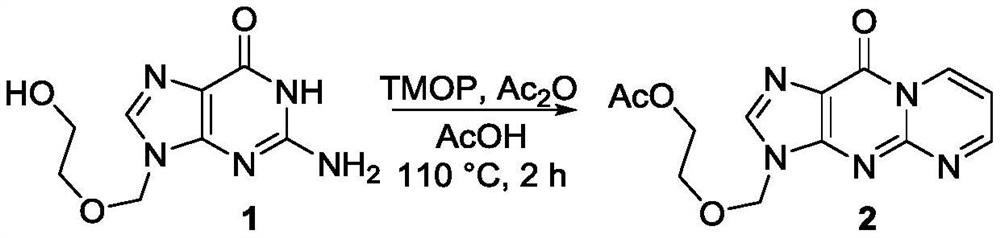
[0062] Preparation of Compound 2:
[0063]
[0064] The operation is as follows: Acyclovir 1 (22.5mg, 0.1mmol), Ac 2 O (95uL, 1.0mmol), AcOH (0.4mL) and 1,1,3,3-tetramethoxypropane (20uL, 0.12mmol) were sequentially added to a 25mL glass sealed tube, the cap in the sealed tube was tightly closed, and the The reaction mixture was stirred at 110° C. for 2.0 hours in a parallel reactor. After the reaction, cool to room temperature, transfer the reaction solution to a 25mL round-bottomed flask, evaporate and concentrate the reaction solution in a vacuum diaphragm pump with a rotary evaporator in a water bath at 30°C for 5min, spin the reaction solution to dryness, and pass it directly The target product was separated and purified by column chromatography, using THF as the eluent, and then evaporated and concentrated into a solid to obtain product 2 (27.0 mg, 89% yield). Yellow solid, mp: 133–135°C. 1 HNMR (500MHz, CDCl 3 :AcOH-d 4 =18:1)δ9.42–9.38(m,1H),8.94(s,1H),8.06(d,J=...
Embodiment 2
[0066] The preparation of compound 4:
[0067]
[0068] The operation is as follows: Ganciclovir 3 (25.5mg, 0.1mmol), Ac 2 O (95uL, 1.0mmol), AcOH (0.4mL) and 1,1,3,3-tetramethoxypropane (20uL, 0.12mmol) were sequentially added to a 25mL glass sealed tube, the cap in the sealed tube was tightly closed, and the The reaction mixture was stirred at 110° C. for 2.0 hours in a parallel reactor. After the reaction, cool to room temperature, transfer the reaction solution to a 25mL round-bottomed flask, evaporate and concentrate the reaction solution in a vacuum diaphragm pump with a rotary evaporator in a water bath at 30°C for 5min, spin the reaction solution to dryness, and pass it directly Separation and purification by column chromatography, with THF as the eluent, the target product was isolated, and then the target product was evaporated and concentrated into a solid to obtain product 4 (27.5 mg, 73% yield). Yellow solid, mp: 120–122°C, 1 HNMR (500MHz, CDCl 3)δ9.42(d,J=6...
Embodiment 3
[0070] The preparation of compound 6:
[0071]
[0072] The operation is as follows: Penciclovir 5 (25.3 mg, 0.1 mmol), Ac 2 O (95uL, 1.0mmol), AcOH (0.4mL) and 1,1,3,3-tetramethoxypropane (20uL, 0.12mmol) were sequentially added to a 25mL glass sealed tube, the cap in the sealed tube was tightly closed, and the The reaction mixture was stirred at 110° C. for 2.0 hours in a parallel reactor. After the reaction, cool to room temperature, transfer the reaction solution to a 25mL round-bottomed flask, evaporate and concentrate the reaction solution in a vacuum diaphragm pump with a rotary evaporator in a water bath at 30°C for 5min, spin the reaction solution to dryness, and pass it directly The target product was separated and purified by column chromatography, using THF as the eluent, and then evaporated and concentrated into a solid to obtain product 6 (32.5 mg, 87% yield). Yellow solid, mp: 111–113°C. 1 HNMR (500MHz, CDCl 3 )δ9.46(dd, J=7.1and 1.7Hz, 1H), 8.96(t, J=2.8H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com