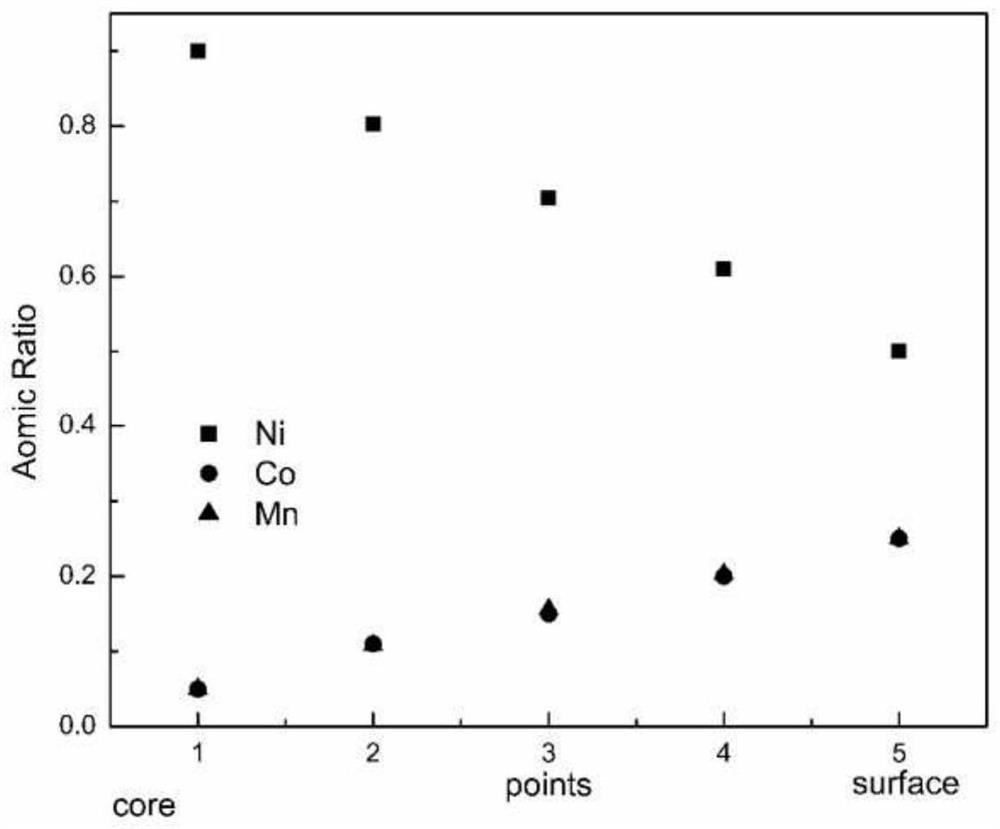
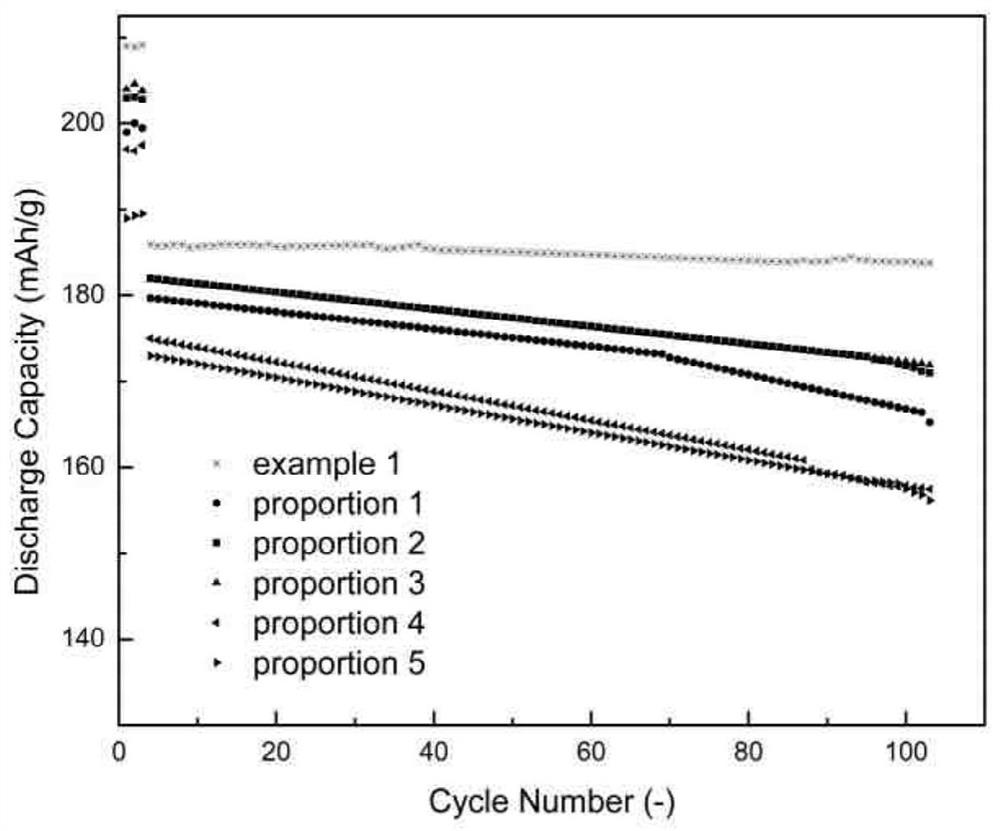
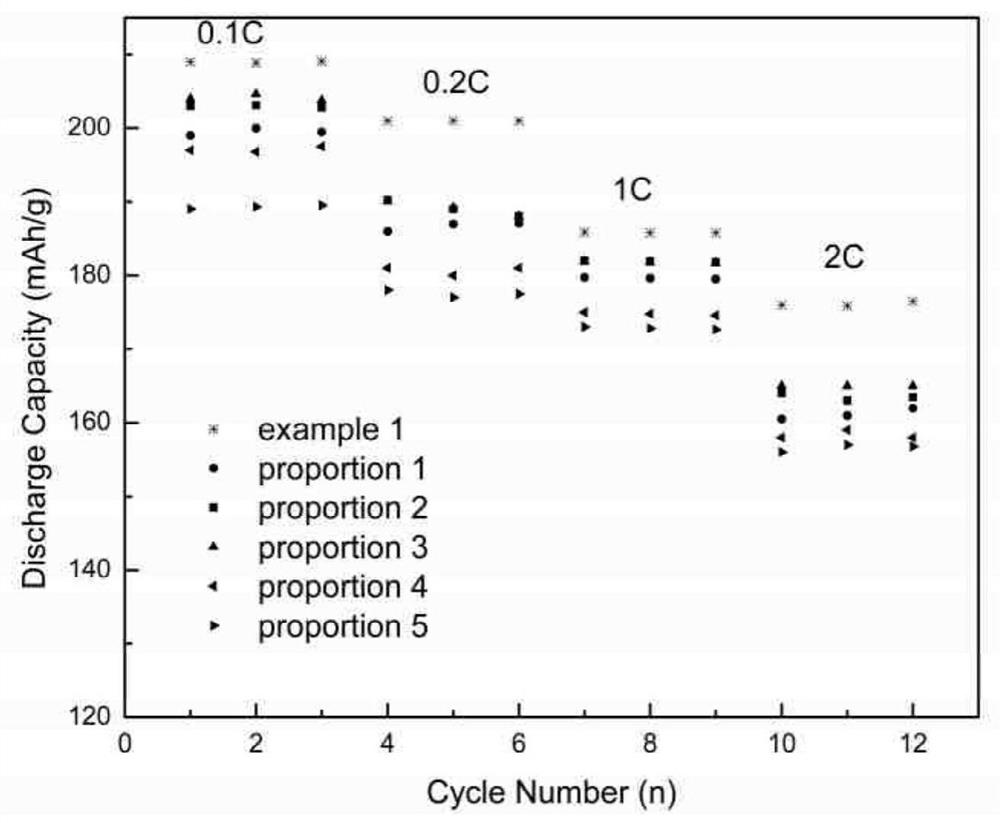
Preparation method of full-concentration gradient positive electrode material precursor, full-concentration gradient positive electrode material and preparation method of full-concentration gradient positive electrode material
A technology of full concentration gradient and positive electrode materials, applied in chemical instruments and methods, battery electrodes, inorganic chemistry, etc., can solve the problems of intermittent control means, slow feedback, uncontrollable, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] The full concentration gradient cathode material prepared in this example is LiNi 0.7 co 0.15 mn 0.15 o 2 , its preparation method comprises the steps:
[0056] (1) The preparation of raw material solution: the sodium hydroxide solution that configures 4mol / L respectively is precipitating agent, and the ammonia solution of 0.825mol / L is complexing agent, nickel sulfate hexahydrate, cobalt sulfate heptahydrate, manganese sulfate monohydrate are dissolved Configure in deionized water to obtain nickel-rich metal salt solution A (Ni:Co:Mn=9:0.5:0.5) and nickel-poor metal salt solution B (Ni:Co:Mn =5:2.5:2.5).
[0057] (2) Synthesis of precursors: Prepare an alkaline base liquid with a pH of 12.00 (sodium hydroxide configuration), an ammonia concentration of 0.9 mol / L, and a volume of 20 L in the reaction kettle, and continuously inject nitrogen gas for 20 minutes to remove the dissolution of the base liquid Oxygen, and keep the reactor sealed and slightly positive pres...
Embodiment 2
[0109] The full concentration gradient cathode material prepared in this example is LiNi 0.6 co 0.2 mn 0.2 o 2 , its preparation method comprises the steps:
[0110] (1) Preparation of raw material solution: configure respectively 4mol / L sodium hydroxide solution as precipitant, 0.525mol / L ammonia solution as complexing agent, dissolve nickel sulfate hexahydrate, cobalt sulfate heptahydrate and manganese sulfate monohydrate The nickel-rich metal salt solution A (Ni:Co:Mn=8:1:1) and the nickel-poor metal salt solution B (Ni:Co:Mn=4:3: 3).
[0111] (2) Synthesis of precursors: Prepare an alkaline base liquid with a pH of 11.80, an ammonia concentration of 0.8 mol / L, and a volume of 20 L in the reactor, and continuously feed nitrogen for 20 minutes to remove dissolved oxygen in the base liquid, and keep the reactor Sealed and slightly positive pressure, the reactor does not require inert gas protection throughout the process. The temperature of the reaction system was contr...
Embodiment 3
[0118] The full concentration gradient cathode material prepared in this example is LiNi 0.5 co 0.25 mn 0.25 o 2 , its preparation method comprises the steps:
[0119] (1) Preparation of raw material solution: configure respectively 4mol / L sodium hydroxide solution as precipitant, 0.15mol / L ammonia solution as complexing agent, dissolve nickel sulfate hexahydrate, cobalt sulfate heptahydrate and manganese sulfate monohydrate Configure the nickel-rich metal salt solution A (Ni:Co:Mn=8:1:1) and the nickel-poor metal salt solution B (Ni:Co:Mn=2:4:1) of the same volume of 2mol / L in deionized water 4).
[0120] (2) Synthesis of precursors: Prepare an alkaline base liquid with a pH of 11.80, an ammonia concentration of 0.8 mol / L, and a volume of 20 L in the reactor, and continuously feed nitrogen for 20 minutes to remove dissolved oxygen in the base liquid, and keep the reactor Sealed and slightly positive pressure, the reactor does not require inert gas protection throughout t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com