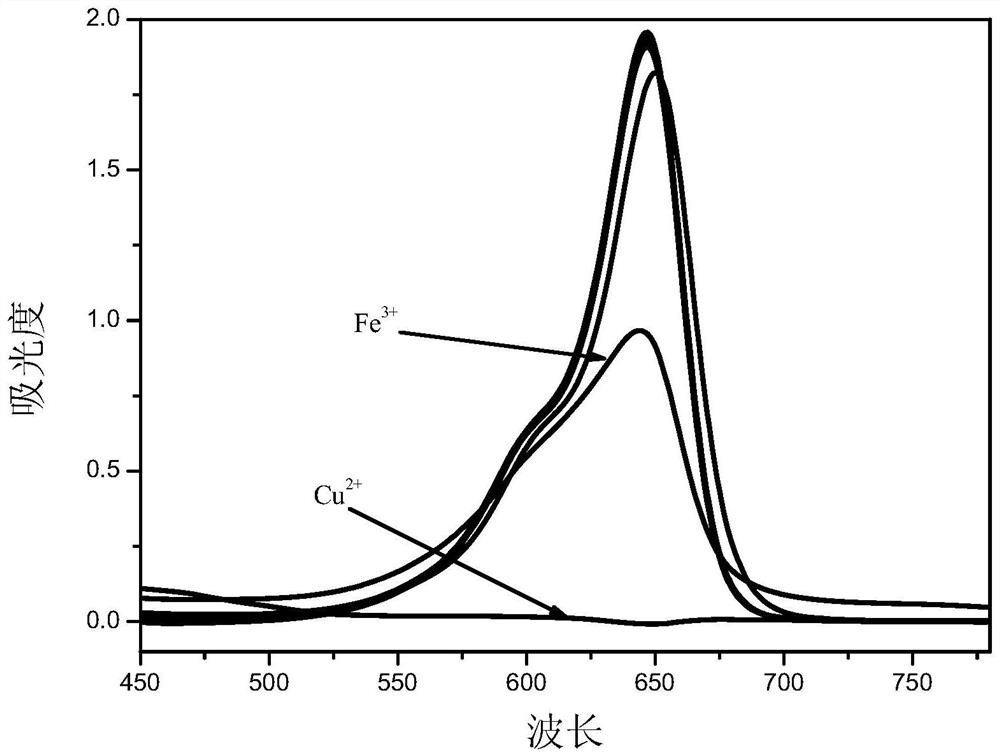
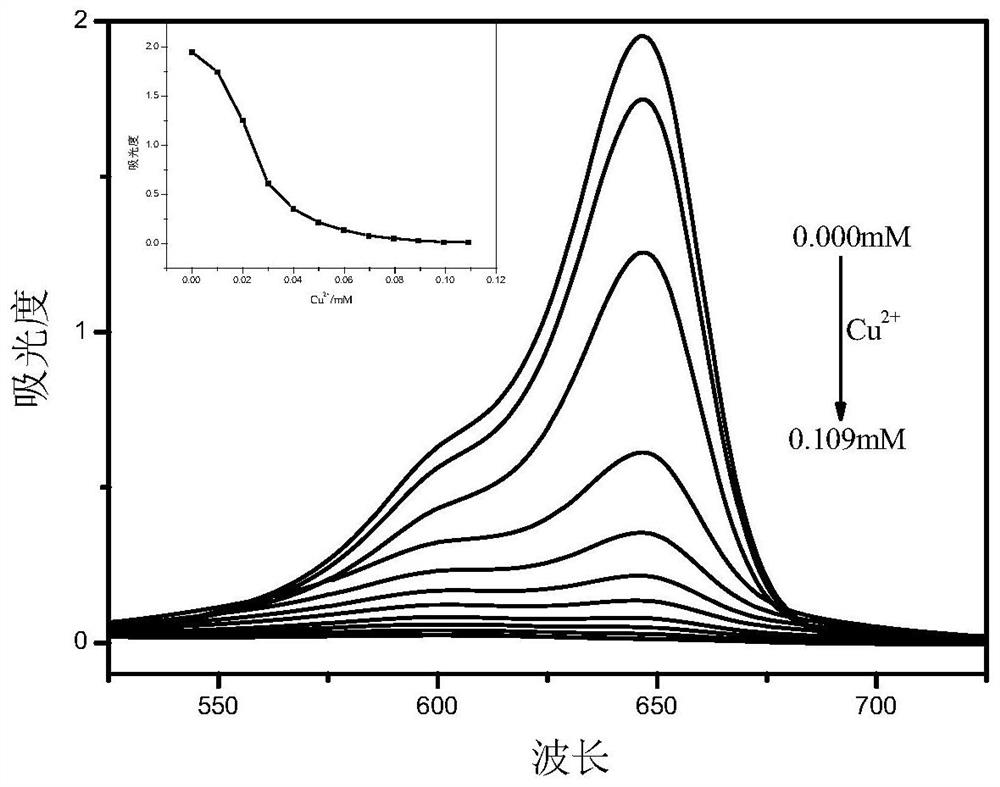
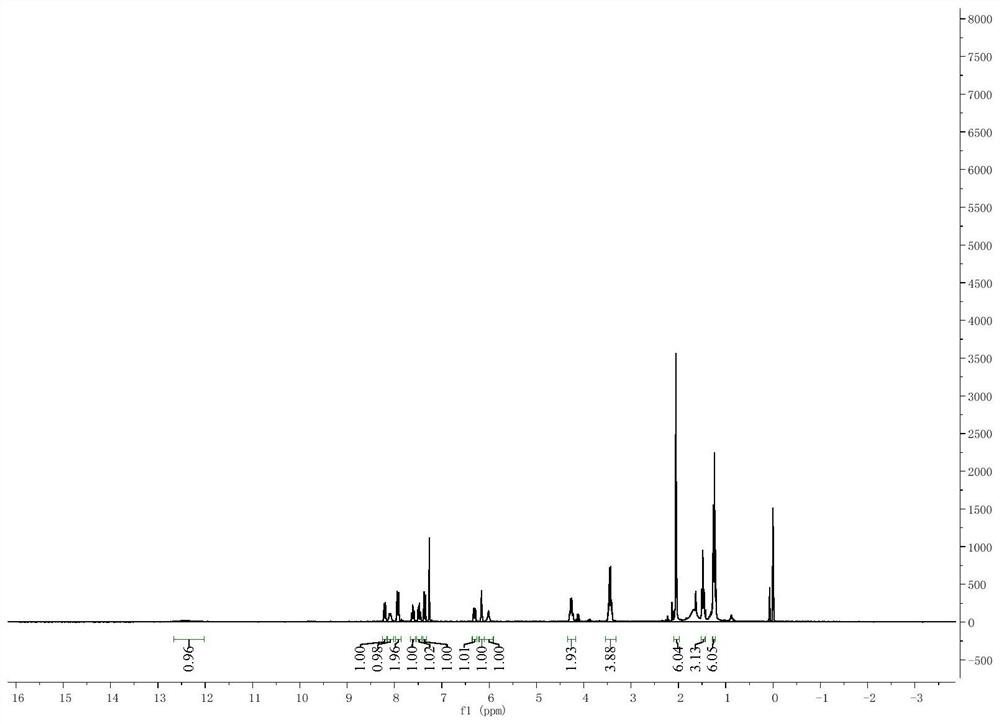
Asymmetric squarylium cyanine colorimetric probe for identifying copper ions and application of asymmetric squarylium cyanine colorimetric probe
A colorimetric probe, asymmetric technology, applied in chemical instruments and methods, measurement of color/spectral characteristics, analysis through chemical reactions of materials, etc., can solve the problems of unstudied selectivity, achieve good selectivity, The effect of high sensitivity and excellent optical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0028] (1) Synthesis of diethoxy squaraine
[0029] Weigh 2.5g (about 8.75mmol) squaraine and dissolve it in 40mL ethanol, heat to 80°C, stir with a constant temperature heating magnetic stirrer, react for 2h, stop the reaction and cool it to room temperature, and remove most of the solvent by rotary evaporation. Continue to add 40mL of ethanol to the round bottom flask, heat to 80°C, stir with a constant temperature heating magnetic stirrer, react for 1h, remove the solvent after the reaction is stopped, repeat the above steps 3 times, separate and purify with a silica gel column to obtain a colorless liquid, namely Crude product of diethoxysquaric acid.
[0030] (2) Synthesis of 3-ethyl-1,1,2-trimethyl-1H-benzo[e]indole
[0031] Weigh 1,1,2-trimethyl-1H-benzo[e]indole 1g (about 5mmol) into a round bottom flask, dissolve it in 50mL toluene, and pour N 2 Protected, and 1.5 g iodoethane (about 10 mmol) was added dropwise, heated to 120 ° C, stirred with a constant temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com