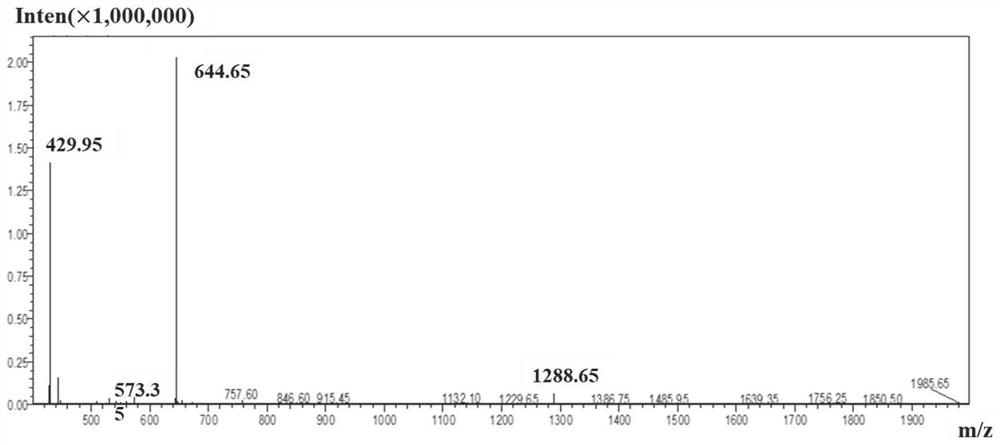
Anti-obesity peptide and application thereof
An obesity and heptapeptide technology, applied in the field of biopharmaceuticals, can solve the problems of anti-obesity peptide verification, and achieve the effect of reducing lipid accumulation and inhibiting differentiation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
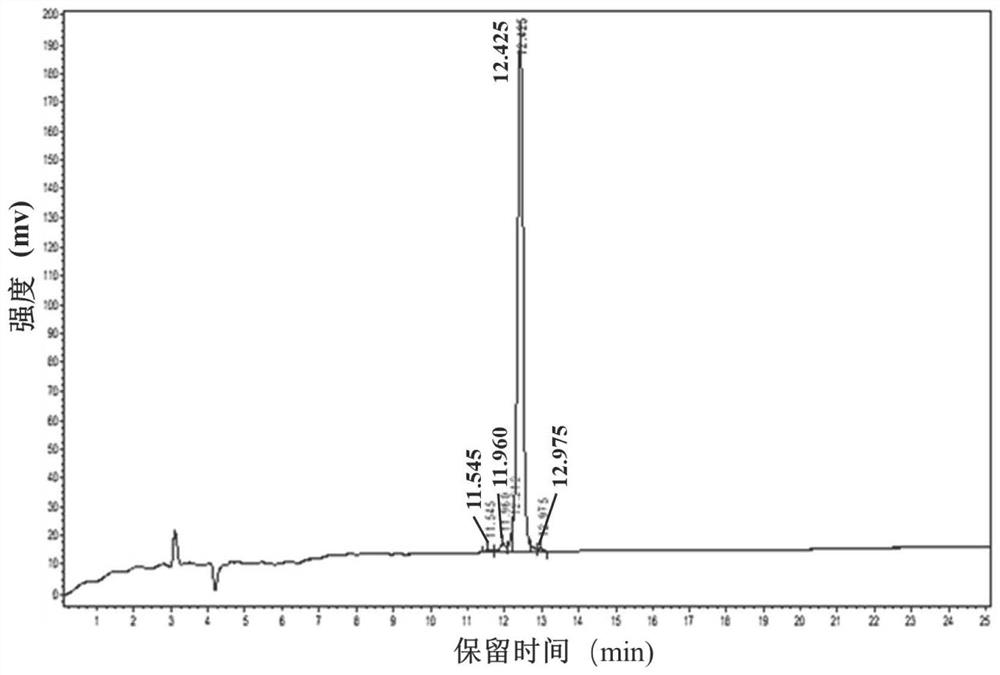
[0097] Add 25 μL of dodecapeptide RGGFLLAMVRAP (RP-12) solution at a concentration of 50 μM to a 96-well black plate, followed by adding 25 μL of pancrelipase solution and 50 μL of 4-methylumbelliferone oleate solution, and incubate at 25°C for 20 minutes in the dark , add 100 μL of sodium citrate solution (0.1M pH=4.2) to stop the reaction, replace the dodecapeptide RGGFLLAMVRAP solution with Tris-HCl buffer as a negative control, and replace the dodecapeptide RGGFLLAMVRAP with Orlistat as a positive control . Tris-HCl buffer was used instead of pancrelipase solution as the background group. After the reaction, record its fluorescence intensity value at the excitation wavelength of 320nm and emission wavelength of 450nm and calculate the inhibition rate of pancreatic lipase.
[0098] Figure 12 Fitting curve for the inhibitory activity of synthetic peptide Arg-Gly-Gly-Phe-Leu-Leu-Ala-Met-Val-Arg-Ala-Pro on pancreatic lipase. Depend on Figure 12 It can be seen that the do...
Embodiment 2
[0100] Add 25 μL of dodecapeptide RGGFLLAMVRAP (RP-12) solution at a concentration of 100 μM to a 96-well black plate, followed by adding 25 μL of pancrelipase solution and 50 μL of 4-methylumbelliferone oleate solution, and incubate at 25°C in the dark for 20 minutes 100 μL of sodium citrate solution (0.1M pH=4.2) was added to stop the reaction, and Tris-HCl buffer was used instead of RGGFLLAMVRAP solution as a negative control, and Orlistat (Orlistat) instead of dodecapeptide RGGFLLAMVRAP was used as a positive control. Tris-HCl buffer was used instead of pancrelipase solution as background. After the reaction, record its fluorescence intensity value at the excitation wavelength of 320nm and emission wavelength of 450nm and calculate the inhibition rate of pancreatic lipase.
[0101] Figure 12 Fitting curve for the inhibitory activity of synthetic peptide Arg-Gly-Gly-Phe-Leu-Leu-Ala-Met-Val-Arg-Ala-Pro on pancreatic lipase. Depend on Figure 12 It can be known that the d...
Embodiment 3
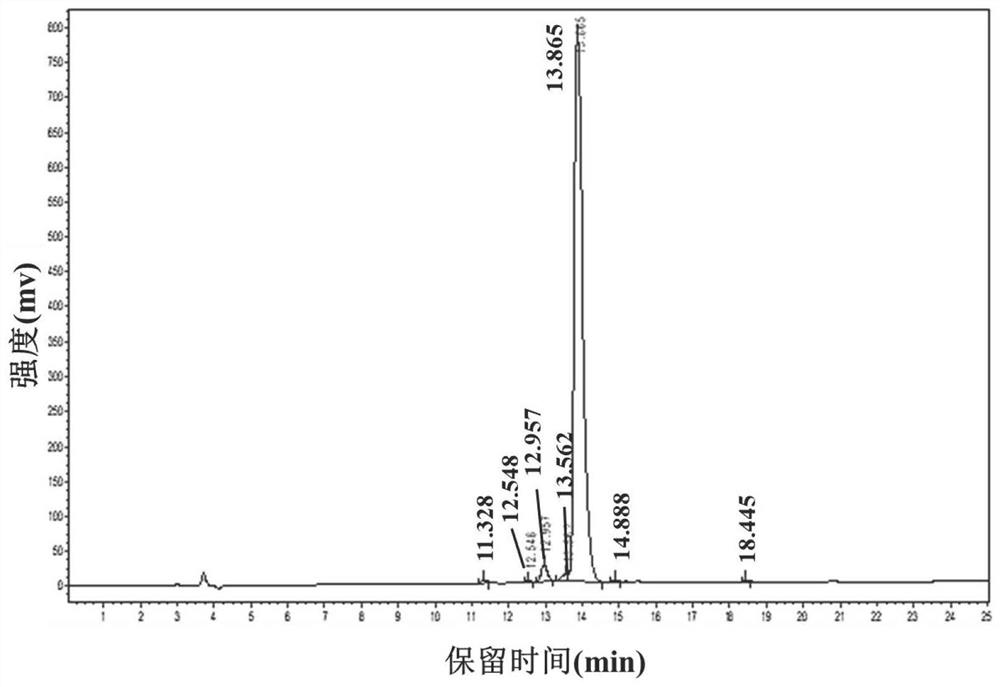
[0103] Add 25 μL of nonapeptide QSAFLHVMY (QY-9) solution at a concentration of 500 μM to a 96-well black plate, followed by 25 μL of pancrelipase solution and 50 μL of 4-methylumbelliferone oleate solution, and incubate at 25°C for 20 minutes in the dark , Add 100 μL of sodium citrate solution (0.1M pH=4.2) to terminate the reaction, use Tris-HCl buffer instead of nonapeptide QSAFLHVMY solution as a negative control, and use Orlistat (Orlistat) instead of nonapeptide QSAFLHVMY as a positive control. Tris-HCl buffer was used instead of pancrelipase solution as background. After the reaction, record its fluorescence intensity value at the excitation wavelength of 320nm and emission wavelength of 450nm and calculate the inhibition rate of pancreatic lipase.
[0104] Figure 13 Fitting curve for the inhibitory activity of synthetic nonapeptide Gln-Ser-Ala-Phe-Leu-His-Val-Met-Tyr on pancreatic lipase. Depend on Figure 13 It can be seen that the nonapeptide QSAFLHVMY with a con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com