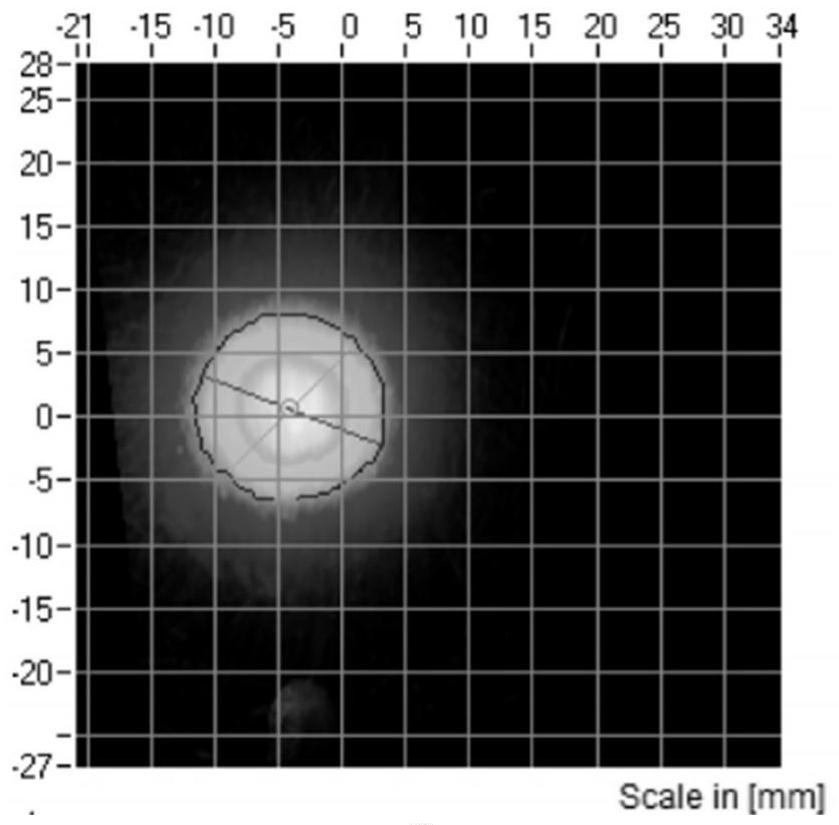
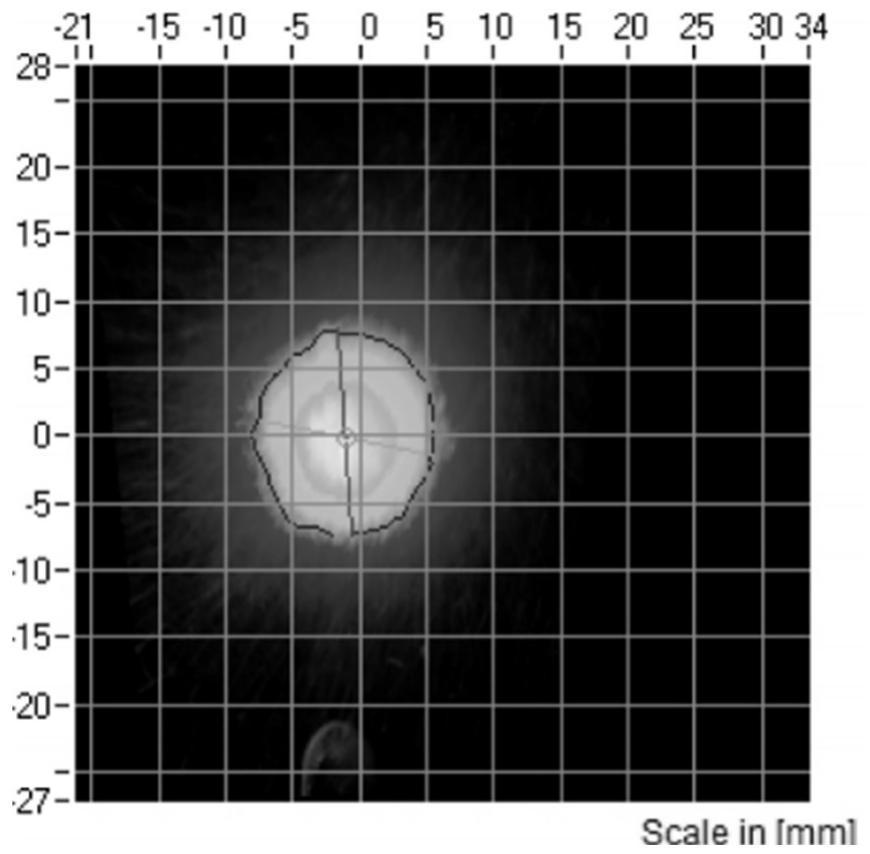
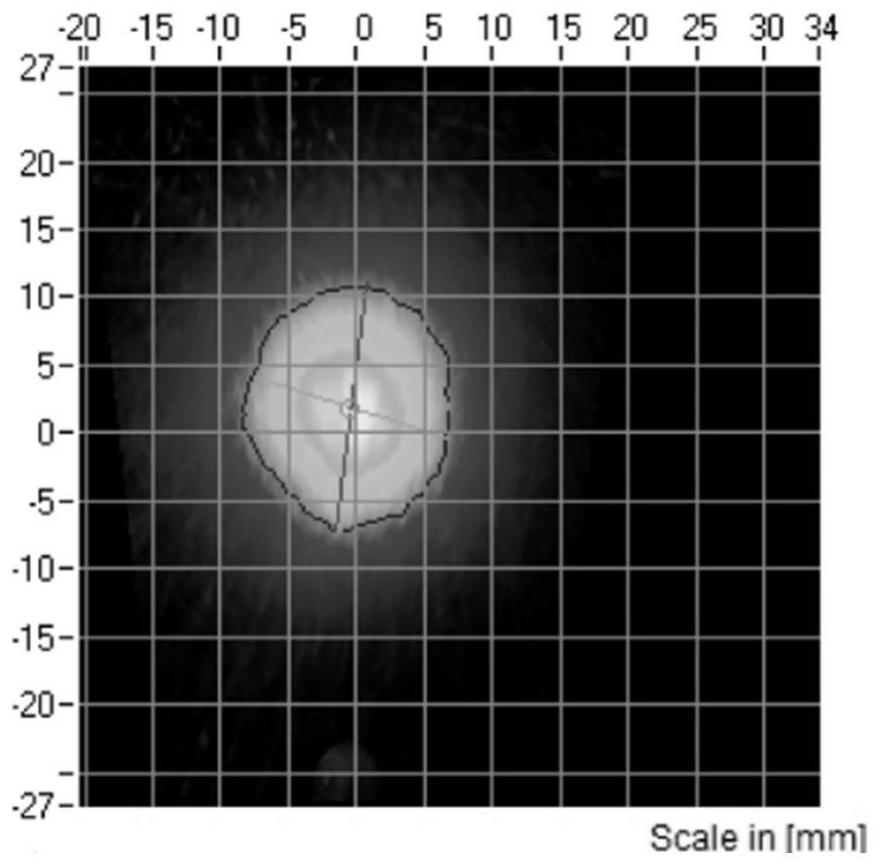
Budesonide nasal spray and preparation method thereof
A nasal spray and surfactant technology, applied in the field of pharmaceutical preparations, can solve problems such as inability to detect spray characteristics, achieve good spray effect, simple preparation method, and good storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Preparation of Bugnadi Nasal Spray
[0050] This embodiment provides a method of preparation of a Budnad nasal spray, and its raw material formulation is shown in Table 1:
[0051] Table 1
[0052] name Prescription composition (g) Budesonide 0.64 Avicel 12.5 Waterless glucose 47.5 Polyshanate 80 0.16 Disodium 0.01 Potassium sorbate 1.20 purified water 937.99, with other components to 1000g
[0053] The preparation of micronized Bud Nida: Using the air current pulverizer to smash the Band Nidal to the particle size below 10 μm or less, to get a micronized Budnad;
[0054] Preparation of A liquid: 47.5 g of water-free glucose, 1.2 g of sorbate, 0.01 g of sorbate, dissolved, spare;
[0055] Preparation of B liquid: Take 50 ml of purified water, add 0.16 g of polysorbate 80, completely dissolve, add 0.64 g of micronized Budnad, stirred and dispersed.
[0056] Preparation of C liquid: Take 200 ml of purified water, add 1...
Embodiment 2
[0058] Example 2 Preparation of Bugnadi Nasal Spray
[0059] This example provides a method of preparation of a Budnad nasal spray, and its raw material formulation is shown in Table 2:
[0060] Table 2
[0061] name Prescription composition (g) Budesonide 0.64 Avicel 12.5 Waterless glucose 47.5 Polyshanate 80 0.16 Disodium 0.01 Potassium sorbate 1.20 purified water 937.99, with other components to 1000g
[0062] The preparation of micronized Bud Nida: Using the air current pulverizer to smash the Band Nidal to the particle size below 10 μm or less, to get a micronized Budnad;
[0063] Preparation of A liquid: 47.5 g of water-free glucose, 1.2 g of sorbate, 0.01 g of sorbate, dissolved, spare;
[0064] Preparation of B liquid: Take 50 ml of purified water, add 0.16 g of polysorbate 80, completely dissolve, add 0.64 g of micronized Budnad, stirred and dispersed.
[0065] Preparation of the C liquid: Take 200 ml of purified water, ad...
Embodiment 3
[0067] Example 3 Preparation of Budnad Nasal Spray
[0068] This embodiment provides a method of preparation of a Budnad nasal spray, and its raw material formulation is shown in Table 3:
[0069] table 3
[0070] name Prescription composition (g) Budesonide 1.28 Avicel 12.5 Waterless glucose 47.5 Polyshanate 80 0.16 Disodium 0.01 Potassium sorbate 1.20 purified water 937.35, with other components to 1000g
[0071] The preparation of micronized Bud Nida: Using the air current pulverizer to smash the Band Nidal to the particle size below 10 μm or less, to get a micronized Budnad;
[0072] Preparation of A liquid: 47.5 g of water-free glucose, 1.20g sorbate, 0.01 g of sorbate, 0.01 g and sodium, 0.01 g of sodium water, 0.01 g and sodium, 0.01 g.
[0073] Preparation of B liquid: Take 50 mL of purified water, add 0.16 g of polysorberry 80, completely dissolved, add 1.28 g of micronized Budnad, stirred and dispersed;
[0074] Preparatio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com