Application of combined medication of bacteroides fragilis and PD-1 and PD-L1 antibodies in treatment of respiratory system tumors
A Bacteroides fragilis, PD-L1 technology, applied in anti-tumor drugs, antibodies, drug combinations, etc., can solve the problem of no PD-1/PD-L1 antibody microecological preparations, etc., and improve the body's anti-tumor immune response. , the effect of prevention and treatment of respiratory system tumors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Preparation of Bacteroides fragilis Live Bacteria Liquid and Inactivated Bacteria Liquid
[0050] Streak inoculation of Bacteroides fragilis ZY-312 strain on blood plate, anaerobic culture for 48h. Observe the colony morphological characteristics, staining characteristics, size, club shape and distribution, etc.
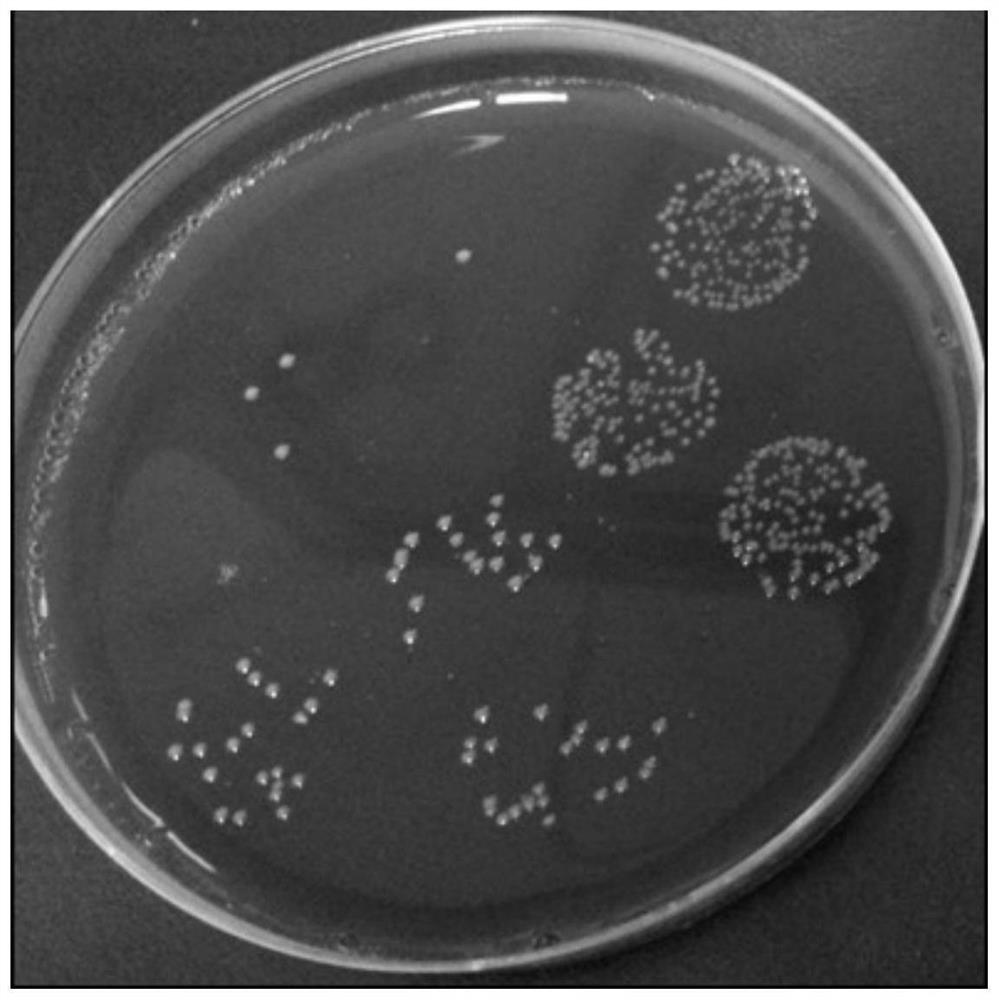
[0051] Colony characteristics: After Bacteroides fragilis ZY-312 was cultured on a blood plate at 37°C for 48 hours, it was slightly convex, translucent, white, smooth, non-hemolytic, and the diameter of the colony was between 1mm and 3mm. See figure 1 .
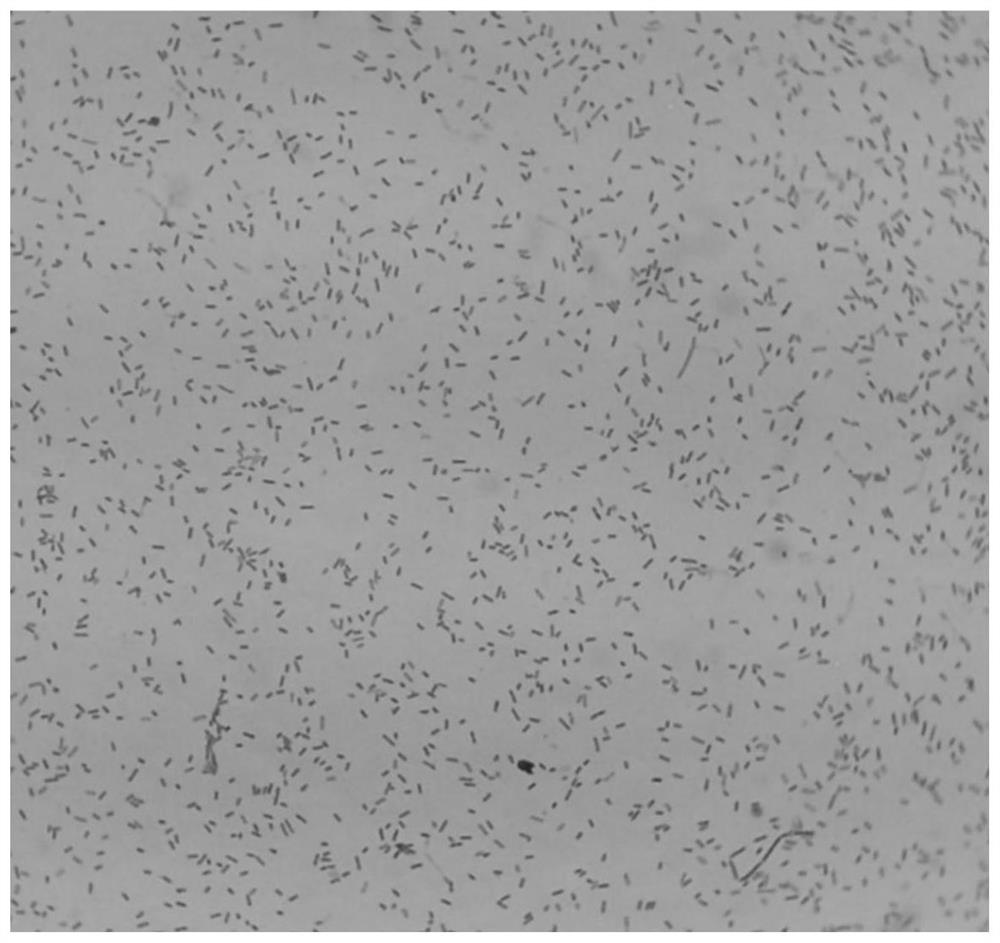
[0052] Morphology under the microscope: Bacteroides fragilis ZY-312 was examined by Gram staining. It is a Gram-negative bacterium with a typical rod shape, blunt rounded ends and dense staining. The uncolored part in the middle of the bacteria is like a vacuole. figure 2 .
[0053] A single colony was selected and inoculated in a plant-derived peptone liquid medium for fermentation and cultiva...
Embodiment 2
[0055] Example 2 Application of Bacteroides fragilis combined with PD-1 antibody in the treatment of non-small cell lung cancer
[0056] 1. Materials and methods
[0057] (1) Experimental grouping and dosing regimen
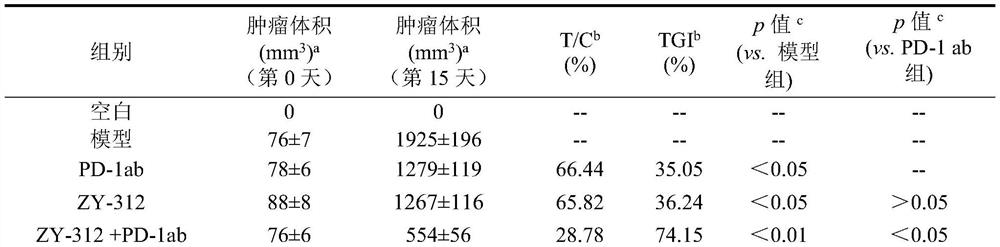
[0058] 70 BALB / c female mice were selected and randomly divided into 7 groups according to the body weight range, namely blank group, model group, ZY-312 group (10 10 CFU / monkey), PD-1 antibody (PD-1 ab) group (purchased from BioXcell, product number BE0146, the same below 200 μg / monkey), ZY-312 live bacteria combined with PD-1 antibody group (10 10 CFU / only), ZY-312 inactivated bacteria group (10 10 CFU / only), ZY-312 inactivated bacteria combined with PD-1 antibody group (10 10 CFU / only), 10 in each group. Except for the blank group, animals in other groups were injected with 1×10 6 LLC cells, tumor volume reaches 50-100mm 3 Dosing in groups was started at D0: starting from D0, animals in the blank group and the model group were orally administered 300 μL ...
Embodiment 3
[0101] Example 3 Application of Bacteroides fragilis combined with PD-L1 antibody in the treatment of non-small cell lung cancer
[0102] 1. Experimental design and process
[0103] Experimental design: 70 BALB / c female mice were selected and randomly divided into 7 groups according to body weight range, namely blank group, model group, ZY-312 group (10 10 CFU / monkey), PD-L1 antibody (PD-L1 ab) group (BE0101, BioXcell 200μg / bird), ZY-312 live bacteria combined with PD-L1 antibody group (10 10 CFU / only), ZY-312 inactivated bacteria group (10 10 CFU / only), ZY-312 inactivated bacteria combined with PD-L1 antibody group (10 10 CFU / only), 10 in each group. Except for the blank group, animals in other groups were injected with 1×10 6 LLC cells, tumor volume reaches 50-100mm 3 Dosing in groups was started at D0: starting from D0, animals in the blank group and model group were orally administered 300 μL of normal saline daily, and intraperitoneally injected with 200 μL of PBS on...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com