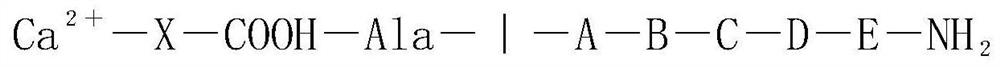Preparation method of polypeptide synthesized amino acid protective agent and DCC product precipitate
A technology for polypeptide synthesis and protective agent, applied in the field of preparation of amino acid protective agent and DCC product precipitation, can solve the problems of difficult precipitation and separation, complex molecular structure, lack of bicyclic imine, etc., and achieves easy precipitation, low price and high precision. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] A preparation method of an amino acid protective agent synthesized by a polypeptide, comprising the following steps:
[0018] Step 1: Take the protective agent X, X is sodium 4-aminobenzenesulfonate, take 1mmol of the protective agent and dissolve it in 10ml of water, fully dissolve, then take 1mmol of alanine to react, the amino group of X combines with the carboxyl end of alanine to form an amide key;
[0019] Step 2: A, B, C, D, E and other amino acids are sequentially connected to the amino terminal of alanine, and at the same time, 1 mmol of DCC carbodiimide is added to react for 2-2.5 hours until the end of the polypeptide chain synthesis;
[0020] Step 3: Take 1 mmol of alanine aminopeptidase, cut off X-alanine with an enzyme, and then add a precipitating agent calcium chloride to react, and remove the protective agent X molecule formed by the precipitation of benzenesulfonic acid group and calcium ion Ca 2+ -X-Ala-NH 2 ;
[0021] A method for preparing the D...
Embodiment 2
[0027] A method for preparing the DCC product precipitation of polypeptide synthesis, comprising the following steps:
[0028] Step 1: adding 5 carbodiimides of 1 mmol to the product of step 3 in claim 1, catalyzing the precipitation of dicyclohexylurea, which was dissolved in water but could not be completely removed in a small amount;
[0029] Step 2: adding a tartaric acid precipitant to the product of step 1, removing dicyclohexylurea through reaction precipitation, filtering the precipitate through a filter, and filtering out the supernatant;
[0030] Step 3: Use a dryer to dry the water to obtain a pure polypeptide product.
[0031] The purity of the prepared polypeptide product is lower than that in Example 1, and the reaction speed and efficiency are lower than that in Example 1, and the cost of the reaction is higher, which is not conducive to a large demand.
Embodiment 3
[0033] A method for preparing the DCC product precipitation of polypeptide synthesis, comprising the following steps:
[0034] Step 1: adding 8 carbodiimides of 1 mmol to the product of step 3 in claim 1, which catalyzes the precipitation of dicyclohexylurea, which cannot be completely removed after being dissolved in water in a small amount;
[0035] Step 2: adding a tartaric acid precipitant to the product of step 1, removing dicyclohexylurea through reaction precipitation, filtering the precipitate through a filter, and filtering out the supernatant;
[0036] Step 3: Use a dryer to dry the water to obtain a pure polypeptide product.
[0037] The purity of the prepared polypeptide product is similar to that of Example 1, and the reaction speed and efficiency are similar to those of Example 1, but the cost of the reaction is very high, which is not conducive to a large demand.
[0038] In summary, when 2 mmol of carbodiimide is added, the precipitated DCC product has the hig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com