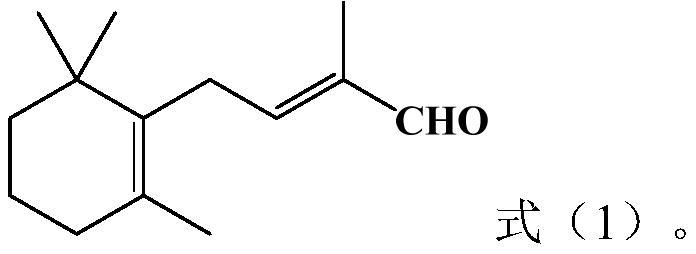
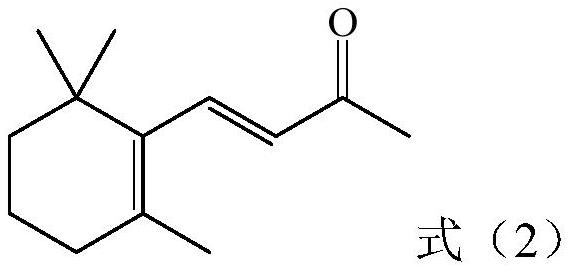
Preparation method of vitamin A intermediate tetradecanal
A technology of tetradecaldehyde and intermediate, which is applied in the field of preparation of vitamin A intermediate tetradecaldehyde, can solve the problems of low yield of vitamin A intermediate tetradecaldehyde and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The preparation method of the vitamin A intermediate tetradecaldehyde provided by the invention comprises the following steps: S1, carrying out Darzens condensation reaction of β-ionone, sodium methylate and methyl chloroacetate to obtain epoxy carboxylate methanol solution; S2, making the Said methanol solution of epoxy carboxylate is subjected to saponification reaction with water to obtain a solution containing sodium epoxy carboxylate; S3, adding non-polar solvent I to the solution containing sodium epoxy carboxylate and then separating the solid and liquid to obtain filter cake and filtrate , adding water to the filtrate and stirring, then standing and stratifying to obtain an upper oil phase and a lower water phase; the upper oil phase is desolvated to obtain unreacted β-ionone, which is directly applied to the Darzens condensation reaction; The lower aqueous phase and the filter cake are decarboxylated together with an aqueous alkali solution, and after the decarb...
Embodiment 1
[0045] S1, using 100g β-ionone, 70.23g sodium methoxide, and 84.65g methyl chloroacetate as raw materials, prepared an epoxy carboxylate methanol solution with an epoxy carboxylate content of 36.8% through Darzens reaction for 8 hours at -30°C 375mL;
[0046] S2. Add 14 mL of water to the methanol solution of epoxy carboxylate, stir at -5°C for 1.5 h to carry out saponification reaction to obtain a solution containing sodium epoxy carboxylate;
[0047] S3, the saponification reaction is completed, add 40mL sherwood oil to the described epoxy carboxylate-containing sodium solution, filter, add 40mL water to the obtained filtrate and stir and leave to stand for stratification to obtain the upper oil phase and the lower water phase; the upper layer The oil phase was concentrated under reduced pressure to remove the solvent to obtain 2.77g oily matter, wherein the content of β-ionone was 97.3%; the lower water phase was added to the filter cake, and the lower filter cake layer was...
Embodiment 2
[0049] S1. Using 100g of β-ionone, 70.23g of sodium methoxide, and 84.65g of methyl chloroacetate as raw materials, 373mL of epoxy carboxylate methanol solution with an epoxy carboxylate content of 37% was prepared by Darzens reaction at 15°C for 3h ;
[0050] S2. Add 18.7mL of water to the methanol solution of epoxy carboxylate, stir at -5°C for 1.5h to carry out saponification reaction to obtain a solution containing sodium epoxy carboxylate;
[0051] S3, the saponification reaction is completed, add 80mL n-pentane to the sodium epoxy carboxylate solution, filter, add 80mL water to the resulting filtrate, stir and leave to separate to separate, to obtain the upper oil phase and the lower water phase; The upper oil phase was concentrated under reduced pressure to remove the solvent to obtain 2.72g oily matter, wherein the content of β-ionone was 97.5%; the lower layer water phase was added to the filter cake, and the lower filter cake layer was stirred and 104g of 40.0% sodiu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com