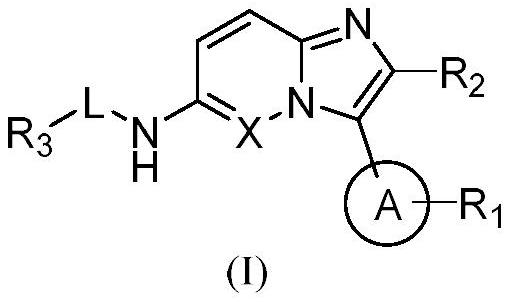
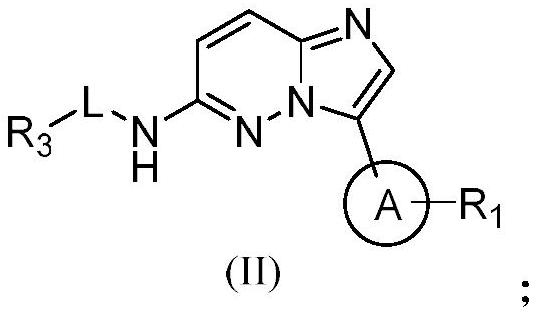
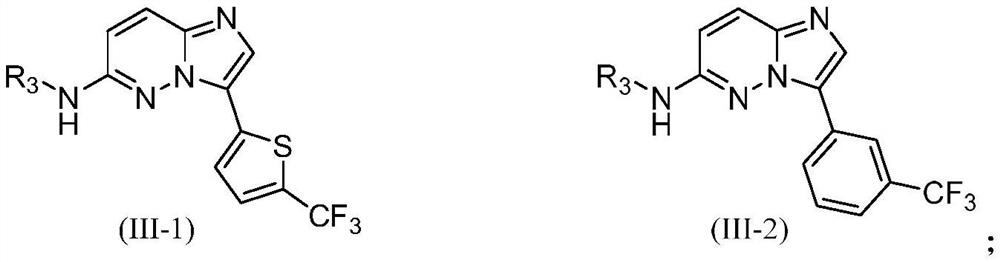PIM kinase inhibitors
An alkyl, selected technology, applied in the field of medicinal chemistry, can solve the problems of low cytostatic activity, low bioavailability, high toxicity, etc., and achieve the effects of high bioavailability, strong drug efficacy, and low toxicity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0216] Example 1: 6-(((1-methylpiperidin-4-yl)methyl)amino)-3-(3-(trifluoromethoxy)phenyl)imidazo[1,2-b] Pyridazine-2-carboxylic acid (Compound 6)
[0217]
[0218] (1) Ethyl 3-bromo-6-chloroimidazo[1,2-b]pyridazine-2-carboxylate
[0219] Dissolve ethyl 6-chloroimidazo[1,2-b]pyridazine-2-carboxylate (1.0 g, 3.92 mmol) in dichloromethane (15 mL), add NBS (1.1 g, 5.88 mmol), and the The reaction system was stirred overnight at room temperature. Water was added to the reaction liquid, and the liquid was extracted and separated with dichloromethane. The organic phase was washed with saturated brine (10 mL), dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the crude product was purified by a reverse-phase column to obtain 3-bromo-6-chloroimidazo[1,2-b]pyridazine- 2-Carboxylic acid ethyl ester (310 mg, yield: 24%), yellow solid. MS(ESI):m / z 305.8[M+H] + .
[0220] (2) Ethyl 6-chloro-3-(3-(trifluoromethoxy)phenyl)imidazo[1,2-b]pyridazine-2-carbox...
Embodiment 2
[0224] Example 2: N-((1-methylpiperidin-4-yl)methyl)-3-(pyridin-3-yl)imidazo[1,2-a]pyridin-6-amine (Compound 7)
[0225]
[0226] (1) 6-Chloro-3-(pyridin-3-yl)imidazo[1,2-a]pyridine
[0227] Referring to the method of step (2) of Example 1, using 3-bromo-6-chloroimidazo[1,2-a]pyridine (200mg, 0.86mmol), pyridin-3-ylboronic acid (105mg, 0.86mmol) as raw materials , the product 6-chloro-3-(pyridin-3-yl)imidazo[1,2-a]pyridine (67 mg, yield: 34%) was obtained as a yellow oily liquid. MS(ESI):m / z 230[M+H] + .
[0228] (2) N-((1-methylpiperidin-4-yl)methyl)-3-(pyridin-3-yl)imidazo[1,2-a]pyridin-6-amine
[0229] Referring to the method in step (3) of Example 1, using 6-chloro-3-(pyridin-3-yl)imidazo[1,2-a]pyridine (67mg, 0.29mmol), (1-methylpiperidine- Starting from 4-yl)methylamine (37.6 mg, 0.29 mmol), N-((1-methylpiperidin-4-yl)methyl)-3-(pyridin-3-yl)imidazol[1,2 -a] Pyridin-6-amine (10.9 mg, yield: 11%), white solid. MS(ESI):m / z 323.2[M+H] + . 1 H NMR (400MHz, DMSO-d ...
Embodiment 3
[0230] Example 3: 1-methyl-4-(((3-(3-(trifluoromethoxy)phenyl)imidazo[1,2-b]pyridazin-6-yl)amino)methyl) Piperidine-4-carboxylic acid (Compound 8)
[0231]
[0232] (1) 6-Chloro-3-(3-(trifluoromethoxy)phenyl)imidazo[1,2-b]pyridazine
[0233] Referring to the method of step (2) of Example 1, using 3-bromo-6-chloroimidazo[1,2-b]pyridazine (800mg, 3.44mmol), (3-(trifluoromethoxy)phenyl) Boronic acid (708.4mg, 3.44mmol) was used as raw material to obtain the product 6-chloro-3-(3-(trifluoromethoxy)phenyl)imidazo[1,2-b]pyridazine (560.2mg, yield: 52%), yellow solid. MS(ESI):m / z314.0[M+H] + .
[0234] (2) 1-(tert-butoxycarbonyl)-4-(((3-(3-(trifluoromethoxy)phenyl)imidazo[1,2-b]pyridazin-6-yl)amino )methyl)piperidine-4-carboxylic acid
[0235] Referring to the method of step (3) of Example 1, using 6-chloro-3-(3-(trifluoromethoxy)phenyl)imidazo[1,2-b]pyridazine (510mg, 1.63mmol), 4 -(Aminomethyl)piperidine-1,4-dicarboxylic acid 1-tert-butyl 4-ethyl ester (466mg, 1.63mmol) a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com