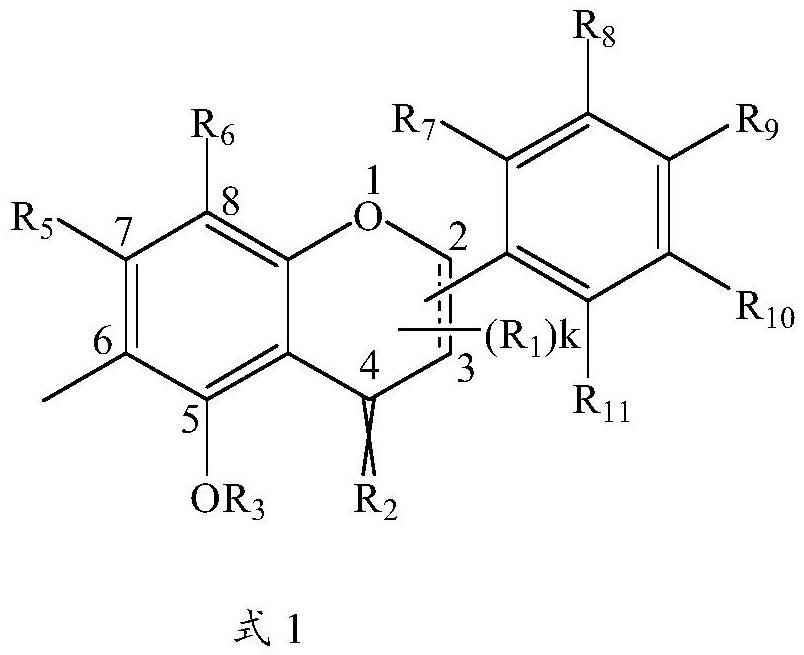
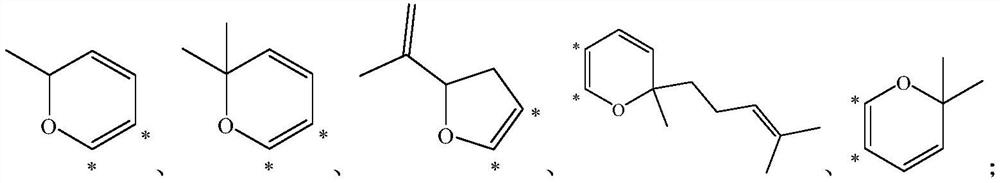
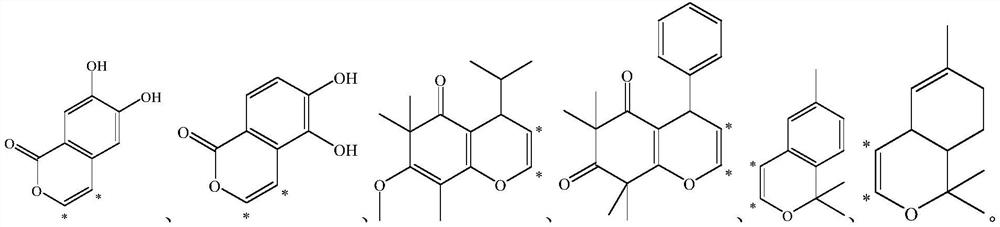
Application of methyl flavonoid compound in preparation of medicine for preventing or treating acute lung injury and/or acute respiratory distress syndrome
A technology for acute respiratory distress and acute lung injury, applied in the field of medicine, can solve problems such as inhibiting pulmonary edema, achieve the effects of inhibiting pulmonary edema, delaying or weakening respiratory distress, and reducing lung wet weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] This example provides a preparation method for compounds 1-1 to 1-12, the specific structure of which is shown in the table below:
[0058] The structure of compound 1-1~1-12 in table 1
[0059]
[0060] Specifically include the following steps:
[0061] Take 20kg of commercially available false eagle's claw roots, crush them, add 120L of ethanol with a volume fraction of 85%, and then extract at 70°C for 3 times, each time for 1 hour, collect and combine the filtrates, and concentrate to obtain an extract. Suspend the extract with water at 60°C to a volume of 5L, and after cooling, extract with petroleum ether, chloroform, ethyl acetate, and n-butanol in sequence. Each solvent is extracted 3 times, and the amount of each solvent is 5L, and the petroleum ether extract is collected. Fr.A, chloroform extract Fr.B.
[0062] Concentrate the petroleum ether extract Fr.A under reduced pressure to remove petroleum ether, then use a silica gel column (column diameter 6cm×h...
Embodiment 2
[0071] This example provides a preparation method of compounds 2-1 to 2-16, the specific structures of which are shown in the table below.
[0072] The concrete structure of table 2 compound 2-1~2-16
[0073]
[0074] It specifically includes the following steps: Weigh 20 kg of dried Spanish clover (Desmodium incanum) whole plant, chop it up, add 6 times the amount of methanol with a volume fraction of 85%, soak and extract at room temperature for 2 times, each time for 24 hours, combine the filtrates, and concentrate to no alcohol smell (solid content 1.45kg), add water to suspend to 5L, extract with cyclohexane, dichloromethane, ethyl acetate, n-butanol successively, each solvent extracts 2 times, each solvent consumption is 5L, collect dichloromethane The extract was concentrated to obtain Fr.A, and the ethyl acetate extract was collected and concentrated to obtain Fr.B.
[0075] Fr.A was separated by a silica gel column (column diameter 6cm×height 60cm, column volume 1...
Embodiment 3
[0084] This example provides a preparation method for compounds 3-1 to 3-32, the specific structures of which are shown in the table below.
[0085] The concrete structure of table 3 compound 3-1~3-32
[0086]
[0087] Concretely include the following steps: take 30 kg of dry root of cultivated Mirabilis jasmine from Tibet in the market, crush it and extract it 3 times with 180 L volume fraction of 75% methanol at 70° C. for 2 hours each time, collect and combine the filtrates, and concentrate to obtain extract (solid content 1.68kg). Suspend the extract with water at 60°C to 5L, and after cooling, extract with petroleum ether, ethyl acetate, and n-butanol in sequence. Each solvent is extracted twice, and the amount of each solvent is 5L. Collect petroleum ether extracts Fr.A, Ethyl acetate extract Fr.B.
[0088]After concentrating Fr.A under reduced pressure to remove the solvent, it was separated on a silica gel column (column diameter 6cm×height 60cm, column volume 1.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com