Preparation method of dipotassium malate
A technology of dipotassium malate and malic acid, which is applied in the field of food processing, can solve the problems of increased solution viscosity, low yield, and unfavorable crystallization, and achieve the effects of increasing production and speeding up the synthesis rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0014] The invention provides a kind of preparation method of dipotassium malate, comprising the following steps:
[0015] S1. Mix malic acid and potassium carbonate in the solution, react at 50-80°C for 0.5-1h, wherein the molar ratio of malic acid and potassium carbonate is 1: (0.9-1.1), after the reaction, separate the solid and liquid , to obtain the filtrate;
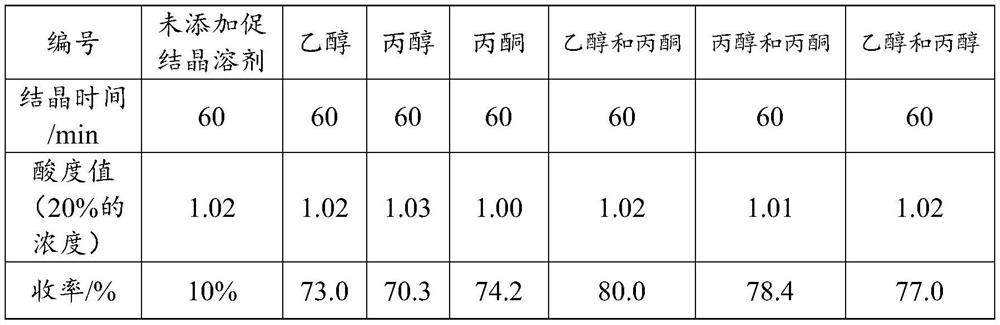
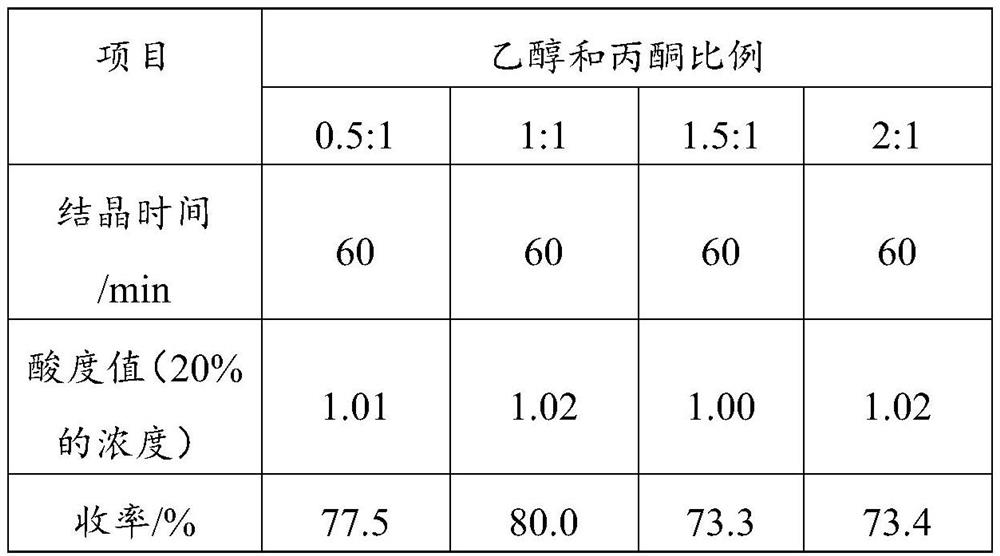
[0016] S2. Concentrate the filtrate of step S1, add a crystallization accelerator, and the crystallization accelerator is any combination of two selected from propanol, ethanol, and acetone, dry after crystallization, and obtain a dipotassium malate product. It is found through research that propanol, ethanol and acetone all have a certain degree of hydrophilicity. After adding the concentrated solution, the crystallization can be significantly promoted, but it is still low. The combination of the two can further significantly increase the yield.
[0017] This application uses malic acid and potassium carbonate as...
Embodiment 1
[0024] The preparation method of the dipotassium malate provided by the present embodiment may further comprise the steps:
[0025] Step A, measure 200ml of distilled water into a beaker, weigh 150g of malic acid, add it to distilled water, stir to dissolve;
[0026] Step B, weigh 148.6g of potassium carbonate, add it to the above solution, and react at 70°C for 60min;
[0027] Step C, after the reaction is completed, filter, concentrate the filtrate to a density of 1.60g / mL, after concentration, it is not suitable for crystallization, add 10mL of crystallization-promoting solvent (4% of the volume of the concentrated solution), leave it for 60min to crystallize, suction filter, and dry to obtain Dipotassium malate product.
[0028] Propanol, ethanol, acetone, ethanol: acetone = 1:1, propanol: acetone = 1:1, ethanol and propanol = 1:1 were used as crystallization-promoting solvents, and no crystallization-promoting solvent was added as a control. Crystallization was carried ...
Embodiment 2
[0036] The preparation method of the dipotassium malate provided by the present embodiment may further comprise the steps:
[0037] Step A, measure 200ml of distilled water into a beaker, weigh 150g of malic acid, add it to distilled water, stir to dissolve;
[0038] Step B, weigh 148.6g of potassium carbonate, add to the above solution, react at 80°C for 60min;
[0039] Step C, after the reaction is completed, filter, and concentrate the filtrate to a density of 1.60g / mL. After concentration, it is not suitable for crystallization. Add 9ml of ethanol and acetone (volume ratio 1:1) (3.5% of the volume of the concentrated solution), and place it for 100min. Crystallization, suction filtration, and drying to obtain dipotassium malate product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com