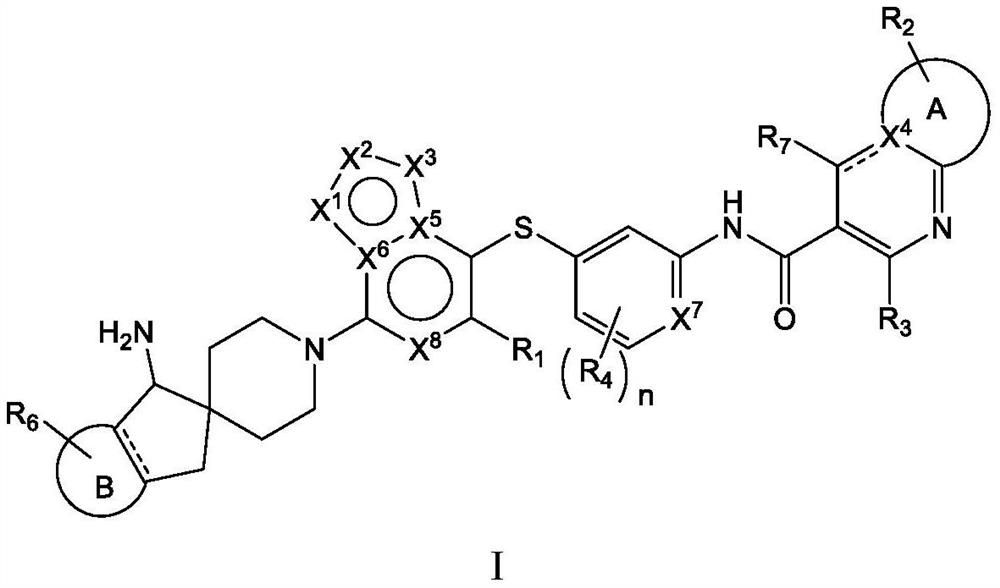
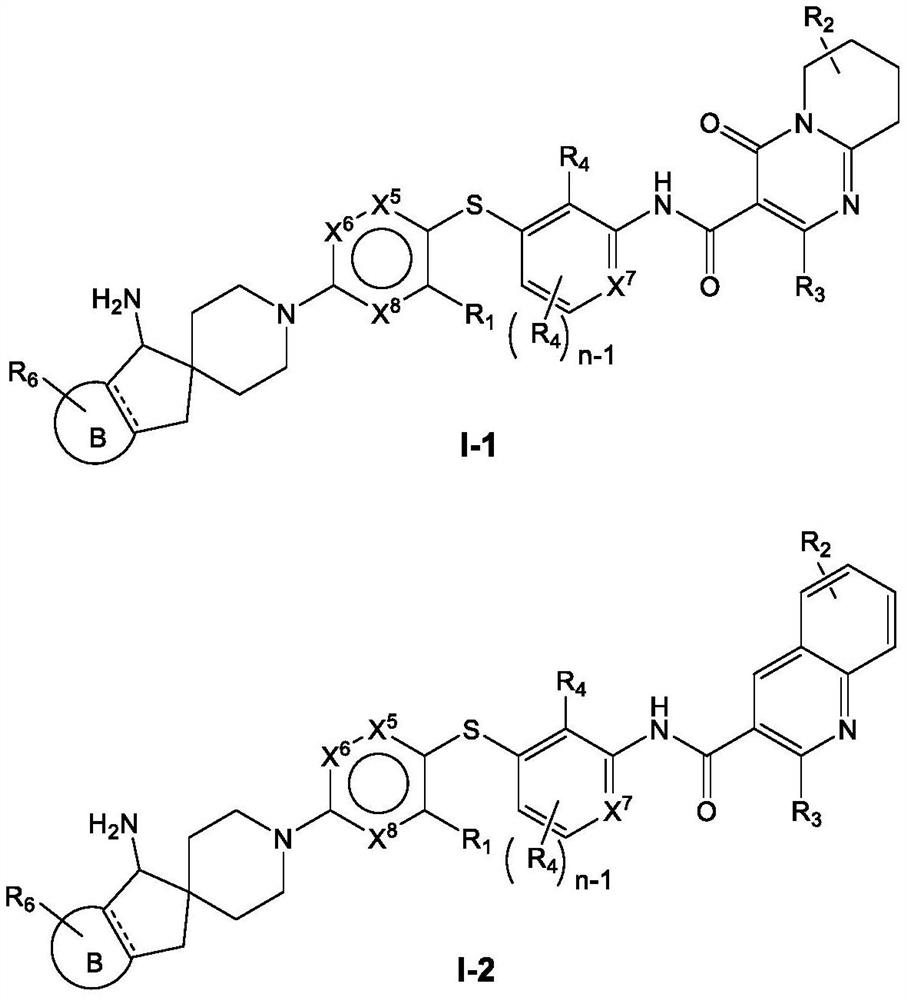
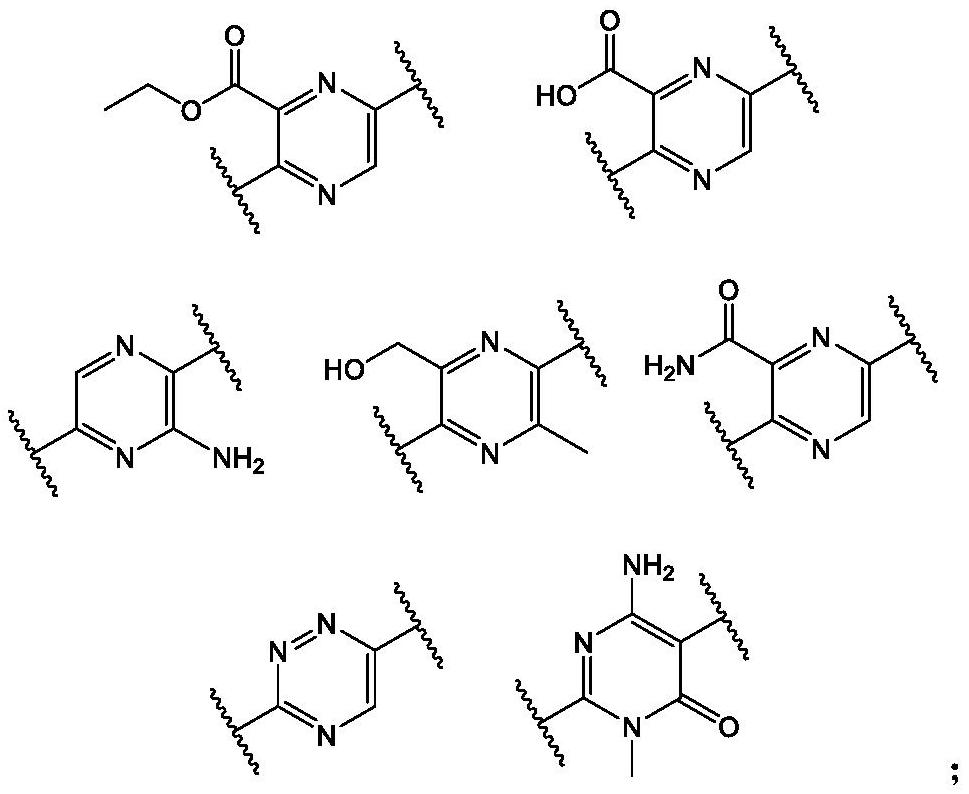
Heterocyclic compound as well as preparation method, pharmaceutical composition and application thereof
A technology of compounds and hydrates, applied in the fields of drug combination, organic chemistry, antitumor drugs, etc., to achieve the effects of excellent biological activity and druggability, superior pharmacological activity and pharmacokinetic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0201] Embodiment 1: the preparation of compound 1
[0202]
[0203] Concrete synthetic route is as follows:
[0204]
[0205] Step 1: Synthesis of Compound 1B
[0206] Compound 1A (104mg, 0.5mmol) was dissolved in dioxane (5ml), and intermediate Q2 (182mg, 1mmol), Pd 2 (dba) 3 (22.73mg, 0.025mmol), Xantphos (14.36mg, 0.025mmol) and DIEA (190mg, 1.5mmol). After heating up to 90°C and stirring for 1 h under nitrogen protection, the reaction mixture was spin-dried and concentrated, and the residue was subjected to silica gel chromatography (eluent: V 二氯甲烷 :V 甲醇 =50:1~10:1) to obtain compound 1B (107 mg, light yellow solid), 75% yield.
[0207] MS(ESI):m / z 287[M+H] + .
[0208] Step 2: Synthesis of Compound 1C
[0209] Compound 1B (100mg, 0.35mmol), DIEA (154mg, 1.2mmol), intermediate Q3 (147mg, 0.7mmol) and HATU (456mg, 1.2mmol) were added to DMF, stirred at room temperature for 12h, TLC showed that after the reaction , the reaction solution was diluted with dichl...
Embodiment 2
[0215] Embodiment 2: the preparation of compound 2
[0216]
[0217] Concrete synthetic route is as follows:
[0218]
[0219] Step 1: Synthesis of Compound 2B
[0220] Compound 2A (1.0 g, 3.6 mmol), Compound Q1 (808 mg, 4.0 mmol) and DIEA (4.6 g, 36 mmol) were added to MeCN (2240 mL), and the resulting solution was stirred at 80° C. for 2 hours. TLC showed the reaction was complete. The mixture was cooled to 25°C, and the Boc 2 O (1.6 g, 7.2 mmol) was added to the solution. The reaction was stirred at 50 °C for 2 hours. TLC showed the reaction was complete. The mixture was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent: petroleum ether / ethyl acetate=1 / 2, V / V) to obtain compound 2B (953 mg, pale yellow solid), with a yield of 48.4%.
[0221] MS(ESI):m / z 547.1[M+H] + .
[0222] Step 2: Synthesis of Compound 2C
[0223] Compound 2C (900mg, 1.6mmol) was dissolved in NMP (10ml), and intermediate Q4 (895mg...
Embodiment 3
[0229] Embodiment 3: the preparation of compound 3
[0230]
[0231] Concrete synthetic route is as follows:
[0232]
[0233] Step 1: Synthesis of Compound 3B
[0234] Compound 3A (6g, 24.80mmol) was dissolved in DMF (50mL), and a DMF solution of DIEA (8.66mL, 49.61mmol) and aminoacetaldehyde dimethyl acetal (2.90g, 27.28mmol) was added dropwise at 0°C. Reaction at room temperature for 2h. After TLC showed that the reaction was finished, water was added and extracted with ethyl acetate, the organic phase was washed with brine, and the organic phase was dried with anhydrous sodium sulfate, and the residue was purified by silica gel column chromatography (eluent: PE:EA=5:1( Volume ratio)) to obtain compound 3B (7.4 g, light yellow solid), yield 91%.
[0235] MS(ESI):m / z310[M+H] + .
[0236] Step 2: Synthesis of Compound 3C
[0237] Compound 3B (5 g, 16.10 mmol) was slowly added to concentrated sulfuric acid (15 mL) in batches under an ice bath, and the temperature w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com