Membrane transporters and uses thereof
A protein, recombinant cell technology, applied in the field of composition of molecules transported across biological membranes, can solve problems such as the biochemical pathway of photosynthesis that has not been described
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
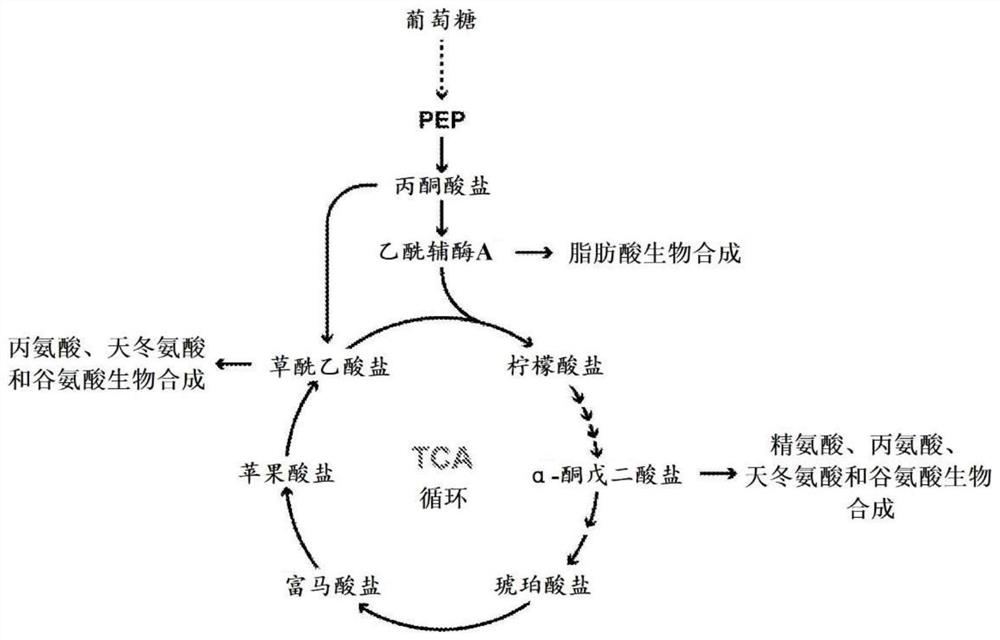
[0257] Example 1: This gene family encodes a family of carboxylate and phosphorylated carboxylate transporters
[0258] To characterize the transport activity of these representative members of this gene family, the genes were cloned into inducible expression vectors ( Figure 18 ).
[0259] Overall, the transport activity of proteins encoded by eight different members of the UPF0114 gene family was experimentally interrogated. These proteins include 1) the protein encoded by the yqhA gene in Escherichia coli, the complete amino acid sequence of the yqhA gene is shown in SEQ ID NO:1. 2) A protein encoded by the AT4G19390 gene in Arabidopsis thaliana, the complete amino acid sequence of the AT4G19390 gene is shown in SEQ ID NO:2. 3) A protein encoded by the Sevir.4G287300 gene in Setaria, the complete amino acid sequence of the Sevir.4G287300 gene is shown in SEQ ID NO:6. 4) A protein encoded by the GRMZM2G179292 gene in maize, the complete amino acid sequence of the GRMZM2G...
Embodiment 2
[0269] Example 2: Transporters can transport metabolites along and against concentration gradients
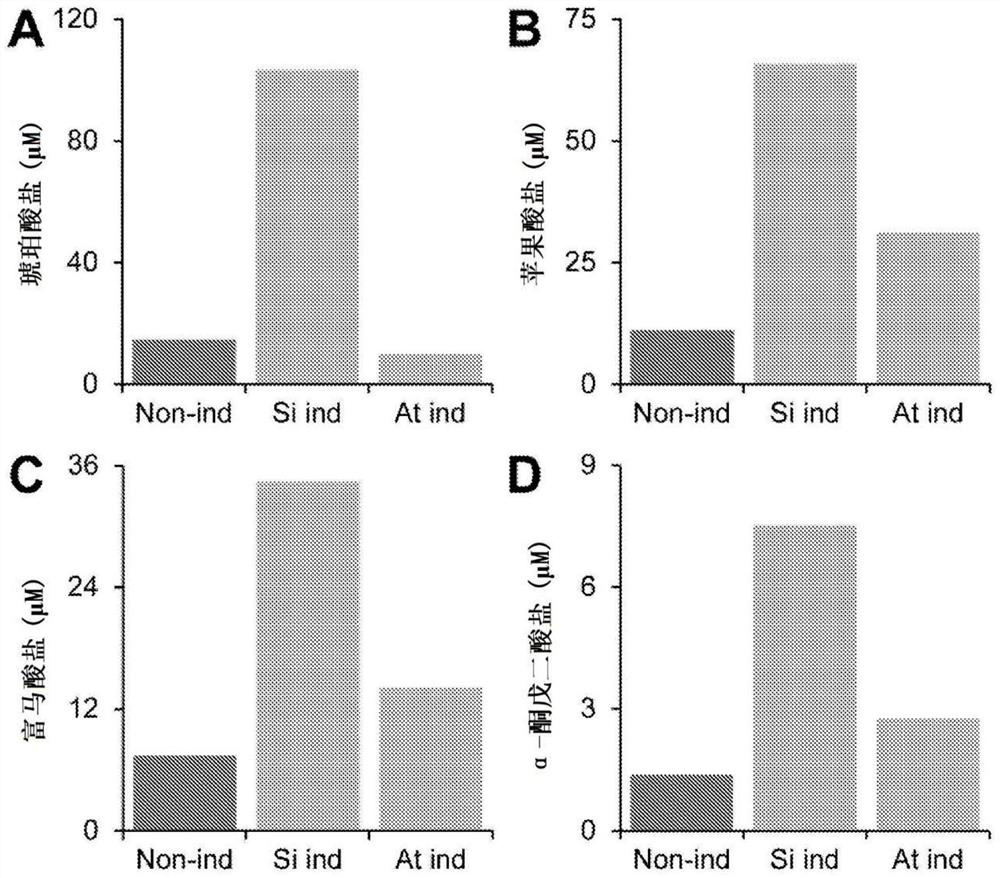
[0270] The intracellular concentration of pyruvate in E. coli was 390 μM. In order to demonstrate that the transporter can export metabolites against the concentration gradient, the experiment described in Example 1 was repeated using the nucleotide sequence of the Sevir.4G287300 gene (amino acid sequence shown in SEQ ID NO: 6) from Setaria officinalis. This time, the M9 glucose growth medium was supplemented with different concentrations of additional pyruvate, resulting in a higher concentration of pyruvate outside the cells than inside the cells. Initial starting concentrations were chosen as 0 μM, 300 μM and 700 μM. In all cases, pyruvate was exported from the cells. In the case of starting concentrations of 300 μM and 700 μM, pyruvate was exported such that pyruvate accumulated to a concentration exceeding the intracellular concentration at three hours ( Figure 7 ).
Embodiment 3
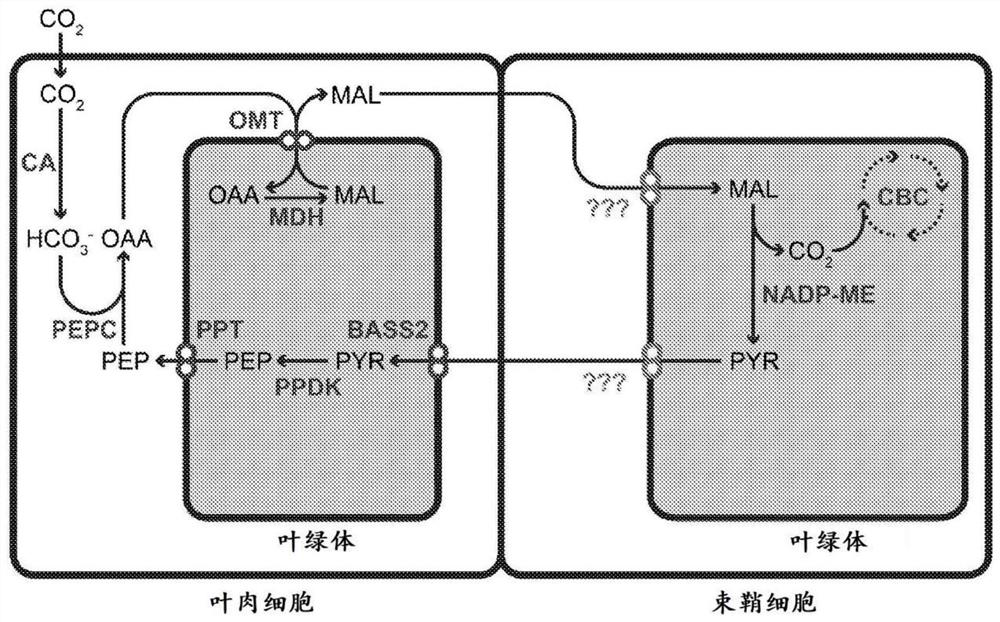
[0271] Example 3: Transport proteins facilitate bidirectional transport of metabolites
[0272] Under aerobic conditions, the dicarboxylate / dicarboxylate transporter dctA is solely responsible for dicarboxylate uptake in Escherichia coli. When the gene encoding dctA is deleted from the E. coli genome, dicarboxylates / dicarboxylates can no longer enter the cell, so E. coli cannot grow on malate as the sole carbon source ( Figure 17 ). However, glucose uptake and subsequent growth on glucose as the sole carbon source were not affected ( Figure 17 ).
[0273]An inducible expression plasmid containing the Sevir.4G287300 gene from Setaria was transformed into a dctA knockout line (ΔdctA). The ΔdctA line containing an inducible expression plasmid was pregrown from cell culture overnight to an OD600 of 0.1 in M9 glucose in a volume of 50 ml. The next day, in two separate flasks, the cell lines were subcultured in M9 glucose to an OD600 of 0.2. Expression of the transported gene...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com