Application of miRNA in preparation of medicine for treating AD, medicine composition and application of medicine composition
A composition and drug technology, applied in the application of drugs, in the field of preparation of therapeutic drugs, can solve problems such as brain tissue damage, Aβ antibody can not reach the therapeutically effective endpoint index, failure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] In order to understand the technical content of the present invention more clearly, the following examples are given in detail.
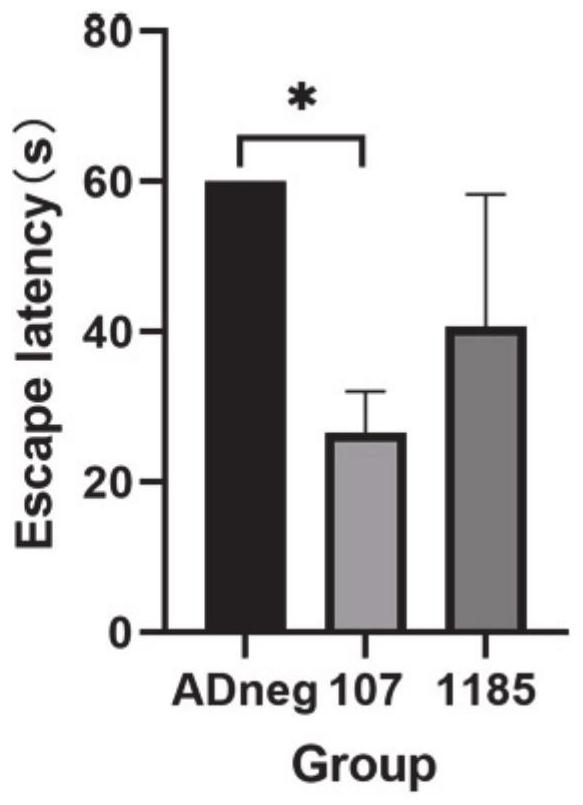
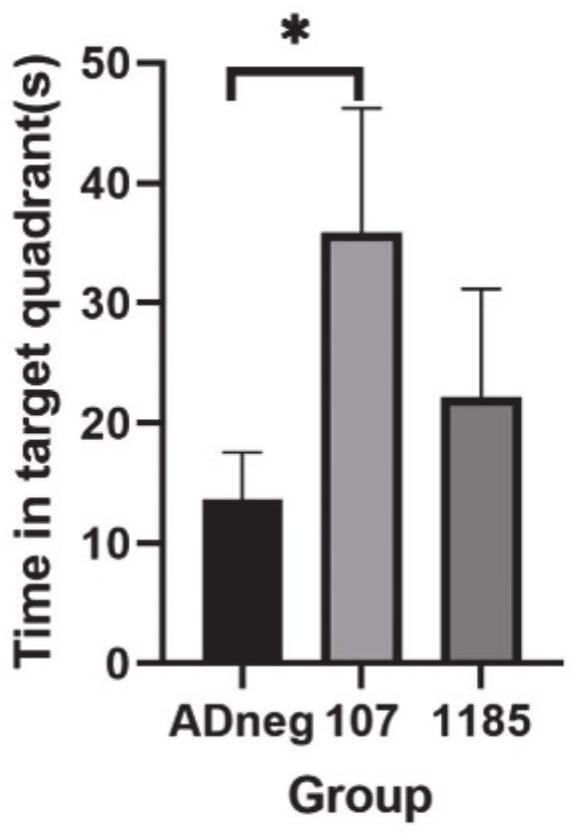
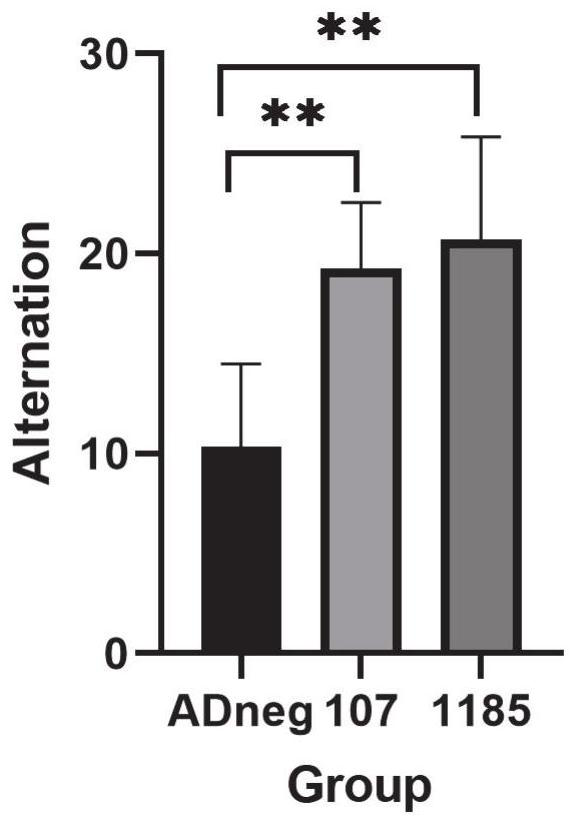
[0019] The applicant's research found that the levels of miR-1185-2-3p and miR-107 were abnormal in the peripheral plasma of early AD patients, and cell experiments confirmed that regulating the levels of these miRNAs in cells can effectively regulate the levels of intracellular BACE1 and Aβ. A major obstacle to combining miRNAs as AD therapeutics is the stability, targeting, blood-brain barrier penetration and biodistribution of exogenous miRNAs in vivo. The adeno-associated virus (AAV) vector is considered as a vector due to its good safety, wide range of host cells, low immunogenicity, long-term stable expression of foreign genes in the body, and the relatively specificity of different serotypes infecting different organs and tissues. One of the most promising gene transfer vectors, widely used in gene therapy and vaccine research worldwid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com