Pharmaceutical composition for treating advanced breast cancer
A technology for advanced breast cancer and its composition, which is applied in drug combinations, antineoplastic drugs, pharmaceutical formulations, etc., can solve the problems of single target and inapplicability, and achieve the effect of significant survival benefit and safe and controllable treatment plan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
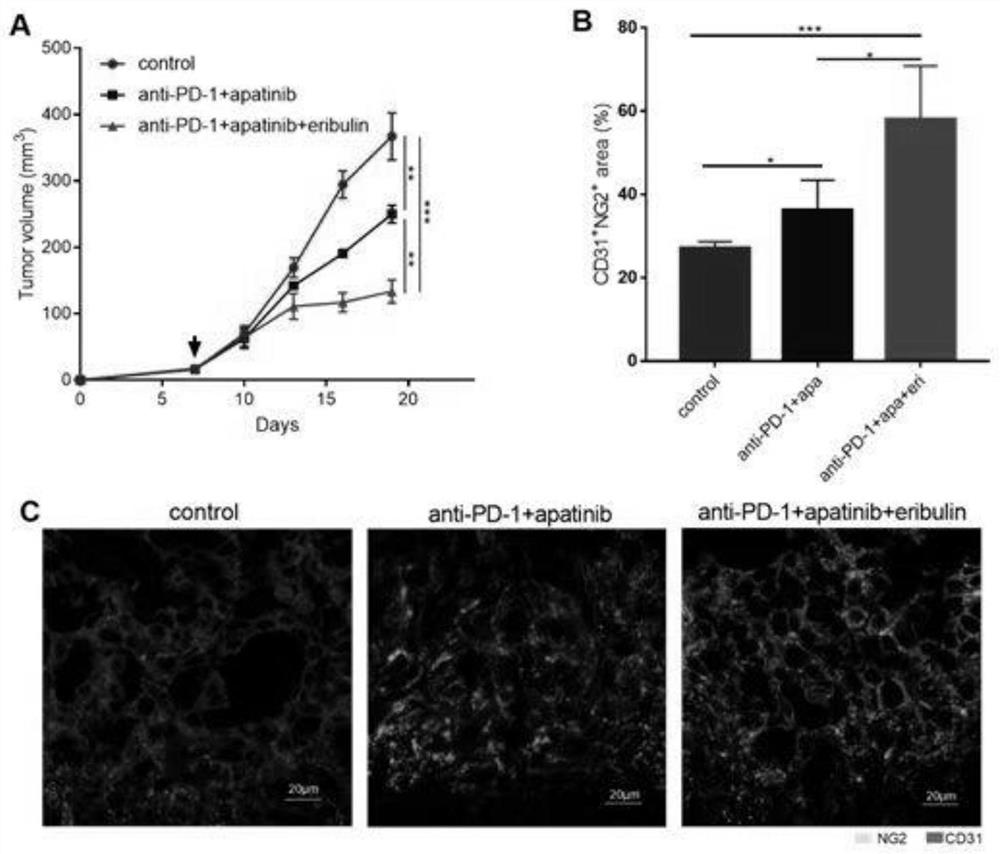
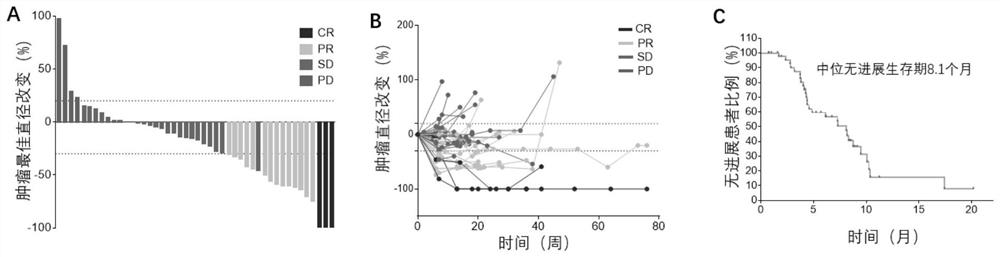
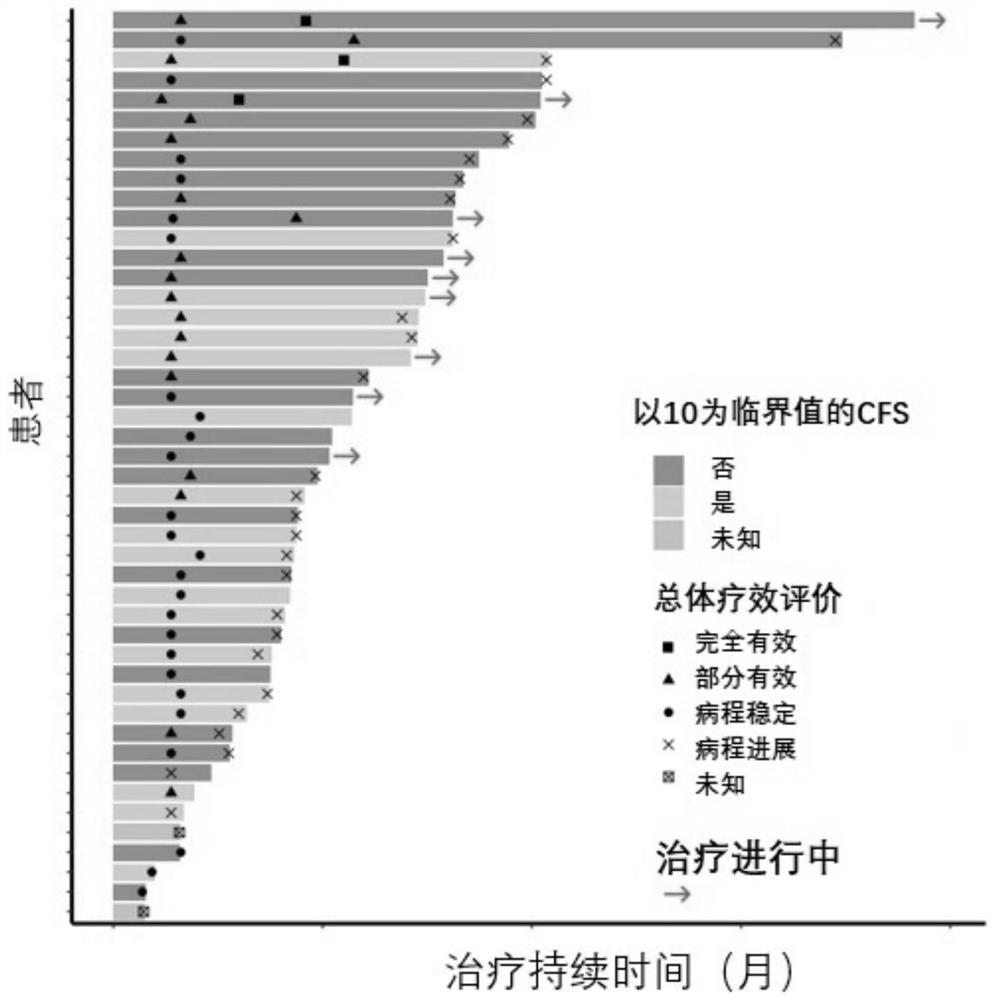
[0108] A pharmaceutical composition for the treatment of advanced breast cancer, the synergistic active ingredients include a therapeutically effective amount of a PD-1 / PD-L1 inhibitor, a therapeutically effective amount of an angiogenesis inhibitor and a therapeutically effective amount of a microtubule inhibitor .
[0109] In the pharmaceutical composition, the synergistic active ingredients are combined in any one of the following ways:
[0110] A therapeutically effective amount (PD-1 / PD-L1 inhibitor + angiogenesis inhibitor + microtubule inhibitor);
[0111] A therapeutically effective amount (PD-1 / PD-L1+ angiogenesis) dual anti-inhibitor and a therapeutically effective amount of a microtubule inhibitor.
[0112] The PD-1 inhibitor is an anti-PD-1 antibody or an antigen-binding portion thereof, preferably an anti-PD-1 monoclonal antibody, more preferably a recombinant humanized anti-PD-1 monoclonal antibody.
[0113] The recombinant humanized anti-PD-1 monoclonal antibo...
Embodiment 2
[0125] Use of PD-1 / PD-L1 inhibitors, angiogenesis inhibitors and microtubule inhibitors in the preparation of pharmaceutical compositions for the treatment of advanced breast cancer.
[0126] PD-1 / PD-L1 inhibitors are anti-PD-1 / PD-L1 antibodies or antigen-binding portions thereof; angiogenesis inhibitors are small molecule kinase inhibitors; microtubule inhibitors are microtubule dynamics inhibitors.
[0127] PD-1 / PD-L1 inhibitor is anti-PD-1 / PD-L1 monoclonal antibody; angiogenesis inhibitor is small molecule tyrosine kinase inhibitor; microtubule inhibitor is halichondrin-type microtubule kinetics inhibitor agent.
[0128] The PD-1 / PD-L1 inhibitor is a recombinant humanized anti-PD-1 / PD-L1 monoclonal antibody; the angiogenesis inhibitor is apatinib or a pharmaceutically acceptable salt thereof; the microtubule inhibitor is Apatinib Ribulin, its pharmaceutically acceptable salts.
[0129] The advanced cancer is advanced melanoma, non-small cell carcinoma, renal cell carcinom...
Embodiment 3
[0131] The pharmaceutical composition for the treatment of advanced breast cancer provided by embodiment 1 or embodiment 2 is the following three kinds:
[0132] Recombinant humanized anti-PD-1 monoclonal antibody, commonly known as camrelizumab for injection (trade name ), which was approved for marketing in China in May 2019. Previous research data shows that the recombinant humanized anti-PD-1 monoclonal antibody injection developed by Hengrui Medicine has comparable in vivo efficacy and safety and may have better anti-tumor clinical application potential.
[0133] Tyrosine kinase inhibitor, commonly known as apatinib mesylate tablets (commodity ), the China Food and Drug Administration approved apatinib for marketing on November 17, 2014. Apatinib has shown corresponding efficacy in the treatment of advanced TNBC phase II clinical trials, which can significantly prolong the progression-free survival (PFS) of patients.
[0134] Microtubule inhibitor, commonly known as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com