Sodium aescinate compound preparation for injection
The technology of aescin sodium and compound preparation, which is applied in the field of medicine, can solve the problems of high incidence of adverse reactions and low incidence of adverse reactions, and achieve the effects of reducing the incidence of adverse reactions, reducing irritation and long storage period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
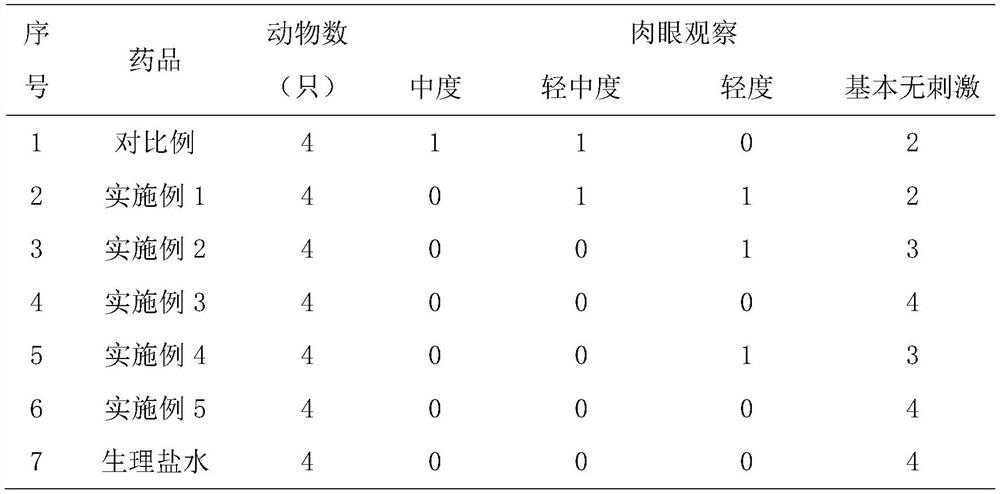
Embodiment 1-4
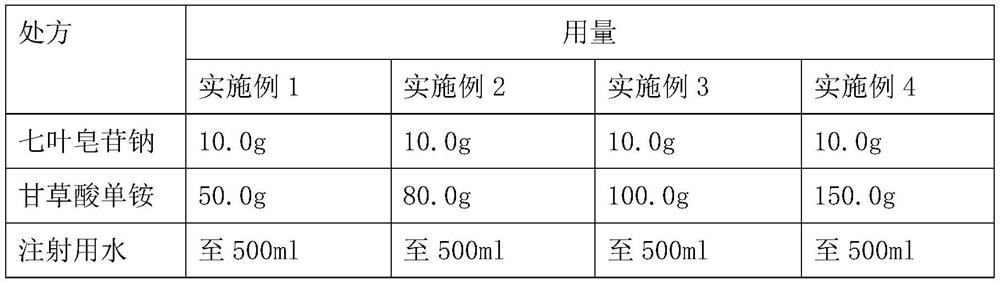
[0017] Sodium aescin for injection per 1000 sticks:
[0018]
[0019] Preparation method:
[0020] 1, ingredients: 10.0g of sodium aesparabonin is added to 400ml of 60 ° C below the injection water to dissolve, dissolve and clarify after adding the prescription amount of glycyrrhizinate monoammonium, continue to stir until dissolved and clarified, make up the injection water to 500ml.
[0021] 2. Filtration: The liquid medicine is first filtered through the 0.45um PES filter membrane, and then filtered by the 0.22um filter membrane.
[0022] 3, filling: the liquid medicine into the cleaning and sterilization of drying treatment of 2ml of the bottle, 0.5ml / stick, and after cleaning and sterilization drying treatment bromide butyl rubber stopper, semi-pressure plug, leaving 2 ~ 3mm of air holes.
[0023] 4. Lyophilization: Put all the filled samples into the lyophilized plate layer, set the lyophilization parameters to start running, and the lyophilization parameters are as foll...
Embodiment 5
[0030] Sodium aescin for injection per 1000 sticks:
[0031] prescription Dosage Aesparabonin sodium 10.0g Diammonium glycyrrhizinate 100.0g Water for injection to 1000ml
[0032] Preparation method:
[0033] 1, ingredients: 10.0g of sodium horse chestnut saponin added to 800ml of 60 ° C below the injection water dissolved, dissolved and clarified after adding 100.0g of ammonium glycyrrhizinate, continue to stir until dissolved and clarified, make up the injection water to 1000ml.
[0034] 2. Filtration: The liquid medicine is first filtered through the 0.45um PES filter membrane, and then filtered by the 0.22um filter membrane.
[0035] 3, filling: the liquid medicine into the cleaning and sterilization of drying treatment of 2ml of cylin bottle, 1.0ml / stick, and after cleaning and sterilization of drying brominated butyl rubber stopper, semi-pressure plug, leaving 2 ~ 3mm of air outlet holes.
[0036] 4. Lyophilization: Put all the filled samples i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com