Application of Ferrostatin-1 in preparation of medicine for relieving inflammatory bowel disease
A technology of inflammatory bowel disease and drugs, applied in the field of medicine, can solve the problems of disappearing curative effect and achieve the effect of improving colitis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
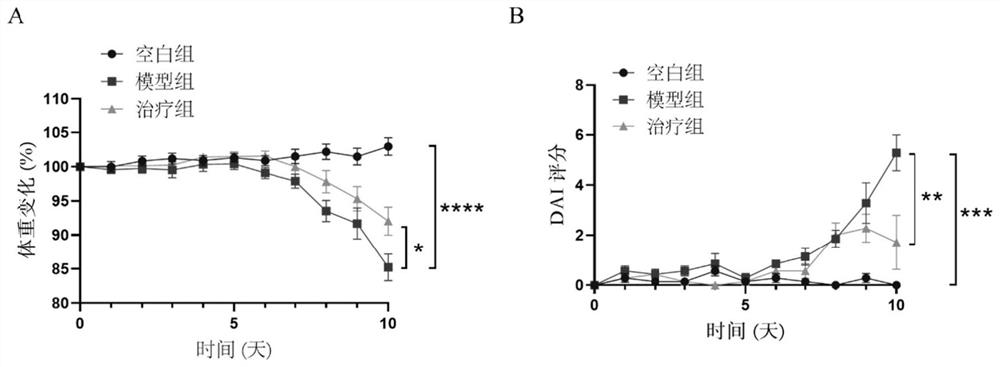
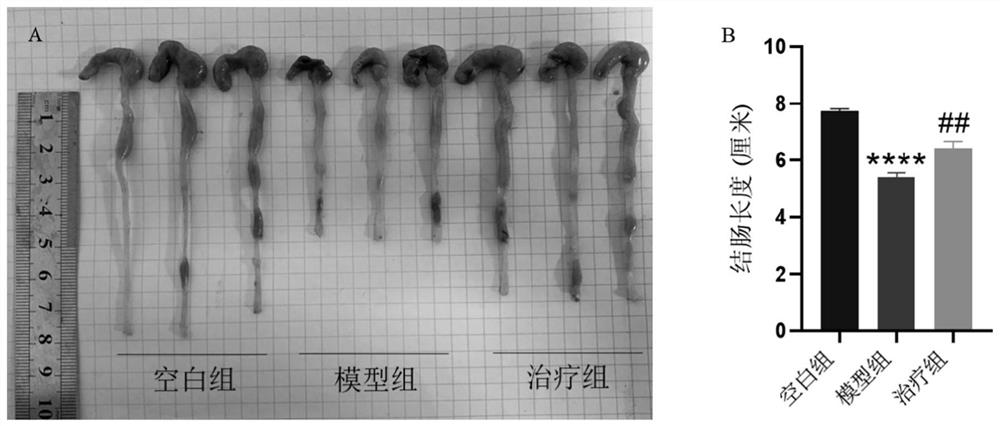
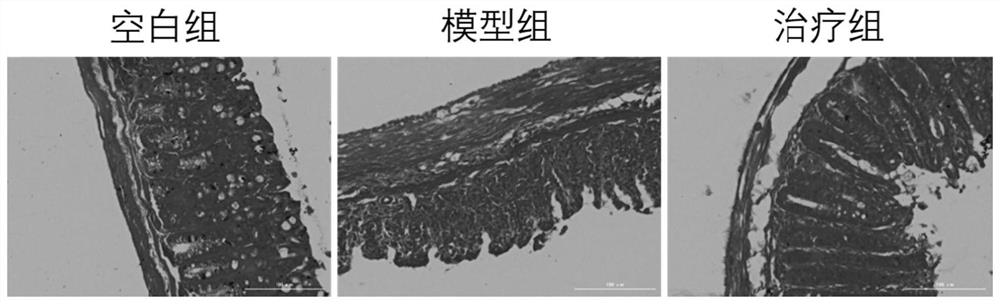
[0106] Example 1 Mouse acute colitis model
[0107] 1. Establishment of a mouse model of acute colitis
[0108] The animal experiments involved in the establishment of the mouse acute colitis model were performed in accordance with the protocols approved by the Animal Ethics Committee of Guangdong Medical University and the committee.
[0109]Female C57 mice aged 6-8 weeks were selected and randomly divided into 3 groups, with 7 mice in each group, namely blank control group (normal diet and drinking water), model group (DSS group, dextran sulphate sodium (DSS) ), treatment group (3% (w / v) DSS+Ferrostatin-1). The blank control group drank ultrapure water normally; in order to induce acute experimental colitis, the mice in the DSS group and the treatment group drank ultrapure water for 3 days, and then freely drank drinking water containing 3% (w / v) DSS for 7 days. The fresh DSS solution was replaced every 1d; the treatment group was intraperitoneally injected with 1 mg / kg of...
Embodiment 2
[0129] Example 2 Fer-1 to H 2 O 2 Effects of treatment on IEC-6 cells
[0130] 1. Cell Culture
[0131] Rat small intestinal crypt epithelial cells (IEC-6 cells) (purchased from Hunan Fenghui Biotechnology Co., Ltd., product number: CL0172) were cultured in 10% (v / v) fetal bovine serum (Gibco, Grand Island) and 1% ( v / v) Penicillin-streptomycin (Gibco, Grand Island) in DMEM medium (Gibco, Grand Island) at 37°C and 5% CO 2 conditions for cultivation.
[0132] 2. Detection of cell viability
[0133] Divide well-grown IEC-6 cells into 5 × 10 cells 3 Cells / well were seeded in 96-well plates, and IEC-6 cells were divided into control group (without any treatment, normal culture), H 2 O 2 Treatment group (H 2 O 2 The final concentration of 400 μM) and H 2 O 2 , Fer-1 co-treatment group (H 2 O 2 The final concentration of Fer-1 was 400 μM and the final concentration of Fer-1 was 5 μM, in H 2 O 2 Before treatment, IEC-6 cells were pretreated with Fer-1 at a final concen...
Embodiment 3
[0137] Example 3 Fer-1 to H 2 O 2 Effects of reactive oxygen species on intracellular lipids in stimulated IEC-6 cells
[0138] IEC-6 cells were grown in DMEM medium containing 10% (v / v) fetal bovine serum and 1% (v / v) penicillin-streptomycin at 37°C and 5% CO 2 conditions for cultivation. Divide well-grown IEC-6 cells into 2.5 × 10 cells 5 The density of cells / well was seeded in 6-well plates, and IEC-6 cells were divided into control group (without any treatment, normal culture), H 2 O 2 Treatment group (H 2 O 2 The final concentration of 400 μM) and H 2 O 2 , Fer-1 co-treatment group (H 2 O 2 The final concentration of Fer-1 was 400 μM and the final concentration of Fer-1 was 5 μM, in H 2 O 2 Before treatment, IEC-6 cells were pretreated with Fer-1 at a final concentration of 5 μM for 30 min), 3 replicates per treatment, 37°C, 5% CO 2 Cultured for 24h under conditions; remove the cell culture supernatant in the 6-well plate, add C11-BODIPY581 / 591 lipid peroxida...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com