Method for preparing 2-fluoro-4-hydroxyaniline
A technology of hydroxyaniline and o-fluoronitrobenzene is applied in the field of preparing 2-fluoro-4-hydroxyaniline by using a continuous flow microchannel reactor, and can solve the difficulties of 2-fluoro-4-hydroxyaniline, poor reaction selectivity, and poor reaction selectivity. Long time and other problems, to achieve the effect of easy process control, efficient response and strong continuity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
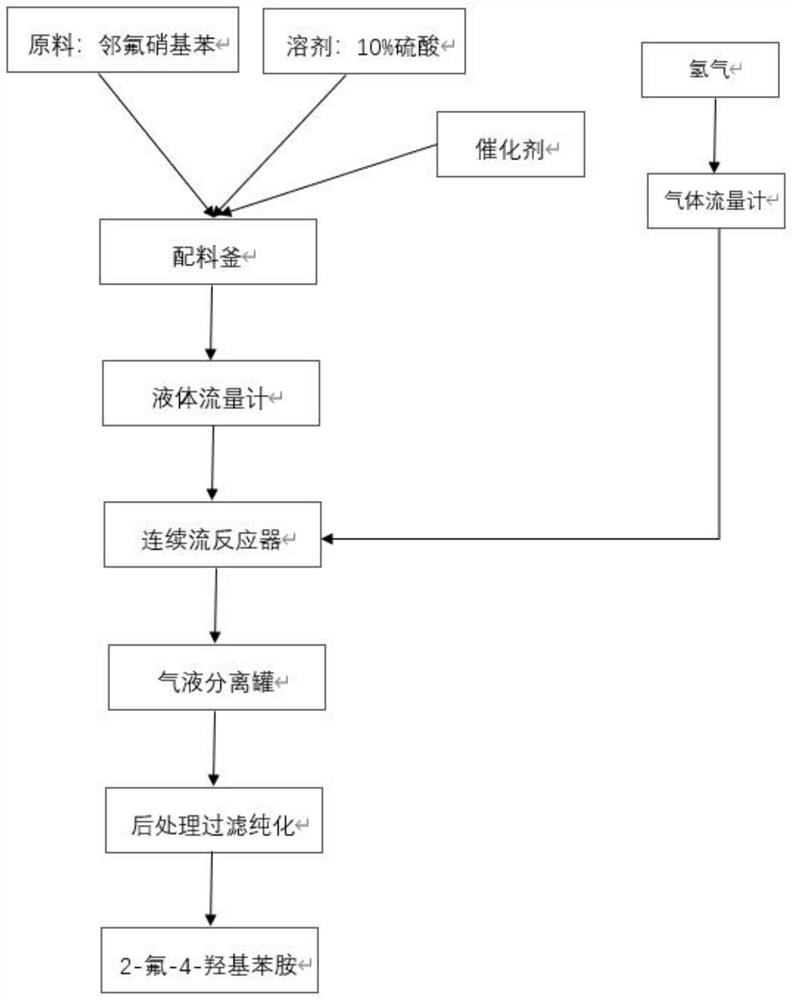
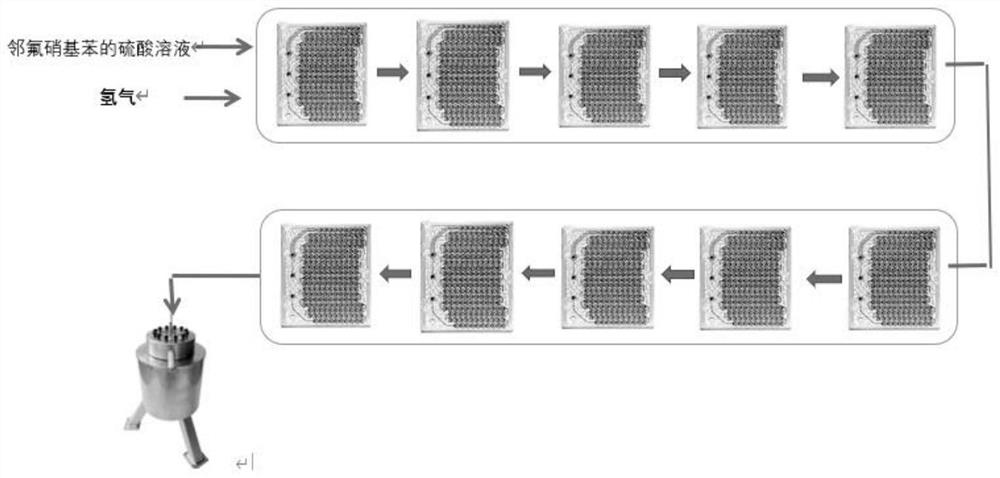
[0036] This embodiment provides a preferred method for preparing 2-fluoro-4-hydroxyaniline. The preparation process and the Corning microchannel reactor reaction process are shown in figure 1 and figure 2 .
[0037] Add 600g (4.25mol) o-fluoronitrobenzene to 2400g of 10% sulfuric acid solution, stir and dissolve; then add 2% platinum carbon 18g, stir, make platinum carbon completely dissolved in sulfuric acid solution, and o-fluoronitrobenzene The base benzene forms a homogeneous solution, that is, the material solution of the present invention.
[0038] The Corning microchannel reactor (consisting of 8 heart-shaped glass modules connected in series) was preheated to a reaction temperature of 60°C, and the configured material solution was injected into the Corning microchannel reactor through a feed pump, while passing through a gas flow meter. Precisely introduced hydrogen into the Corning microchannel reactor to ensure that the molar ratio of o-fluoronitrobenzene and hydr...
Embodiment 2
[0041] Embodiment 2: in the 10% hydrochloric acid solution of 2400g, add 600g (4.25mol) o-fluoronitrobenzene, stir and dissolve; then add 3% ruthenium carbon 24g, stir, make the ruthenium carbon be completely dissolved in the hydrochloric acid solution, It forms a homogeneous solution with o-fluoronitrobenzene, that is, the material solution of the present invention.
[0042] The Corning microchannel reactor (consisting of 6 heart-shaped glass modules connected in series) was preheated to a reaction temperature of 55°C, and the configured material solution was injected into the Corning microchannel reactor through a feed pump, while passing through a gas flow meter. Precisely introduced hydrogen into the Corning microchannel reactor to ensure that the molar ratio of o-fluoronitrobenzene and hydrogen was 1:3.5, the reaction pressure was set to 10bar, and the reaction time in the Corning microchannel reactor was 70s.
[0043] After the reaction, the reaction liquid flows out of ...
Embodiment 3
[0045] Embodiment 3: in the 8% phosphoric acid solution of 2400g, add 600g (4.25mol) o-fluoronitrobenzene, stir and dissolve; then add 3% palladium carbon 9g, stir, make palladium carbon be completely dissolved in 12% nitric acid In the solution, it forms a homogeneous solution with o-fluoronitrobenzene, that is, the material solution of the present invention.
[0046] The Corning microchannel reactor (consisting of 10 heart-shaped glass modules connected in series) was preheated to a reaction temperature of 50°C, and the configured material solution was injected into the Corning microchannel reactor through a feed pump, while passing through a gas flow meter. Precisely inject hydrogen into the Corning microchannel reactor, ensure that the molar ratio of o-fluoronitrobenzene and hydrogen is 1:4, set the reaction pressure to 10bar, and set the reaction time to 120s in the Corning microchannel reactor.
[0047] After the reaction, the reaction liquid flows out of the Corning mic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com