Method for preparing beta-ionone hapten, artificial antigen and antibody
A technology of ionone and artificial antigen, which is applied to the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of hydroxyl compounds, and can solve the technical requirements for the preparation and application of β-ionone haptens, expensive instruments, and β - Ionone is cumbersome to deal with and other problems, and achieves the effects of high purity and yield, easy control, high purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] The preparation method of β-ionone hapten provided in the embodiment of the present invention includes:
[0048] The carboxyl group is directly introduced through the aldehyde group of β-ionone, and the hydroxyl group is generated through the reduction reaction, and the spacer arm with the carboxyl group is obtained.
[0049] The structural formula of the β-ionone hapten preparation method provided in the embodiment of the present invention is as follows:
[0050]
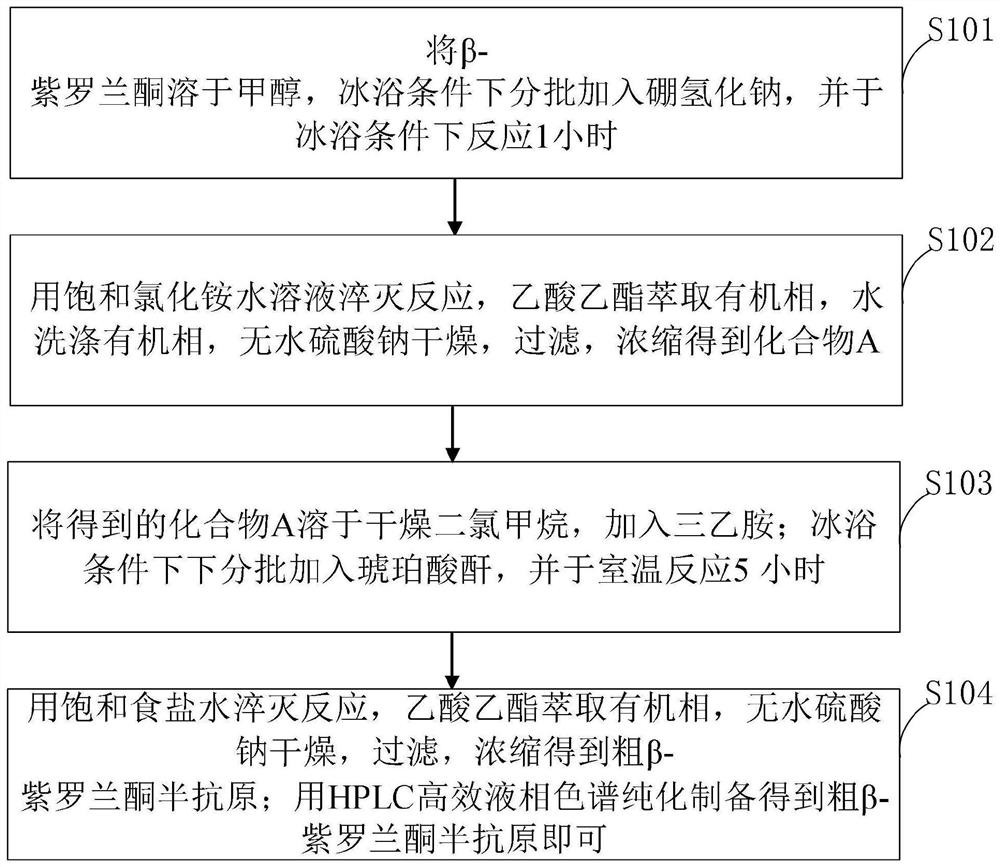
[0051] like figure 1 As shown, the method for preparing β-ionone hapten provided in the embodiment of the present invention comprises the following steps:
[0052] S101, dissolving β-ionone in methanol, adding sodium borohydride in batches under ice-bath conditions, and reacting under ice-bath conditions for 1 hour;
[0053] S102, quench the reaction with saturated aqueous ammonium chloride solution, extract the organic phase with ethyl acetate, wash the organic phase with water, dry over anhydrous sodium...
Embodiment 1
[0078] The invention provides a hapten that can retain the characteristic structure of β-ionone to the greatest extent, and has a certain length of connecting arm and a preparation method of the hapten; artificial antigen and antibody are prepared from the hapten; Application of ionone immunodetection method.
[0079] (1) β-ionone hapten provided by the invention, it has following structural formula:
[0080]
[0081] The β-ionone hapten provided by the present invention is directly introduced into a carboxyl group through an aldehyde group on the β-ionone, so that it can be coupled with a carrier protein to obtain an artificial antigen for immunization. The β-ionone can retain all the chemical structures and characteristic groups of β-ionone to the greatest extent, and is coupled with the carrier protein at the same time. The highlight is the unique structure of β-ionone itself, which is used for subsequent animal immune response, preparation Strongly specific and highly ...
Embodiment 2
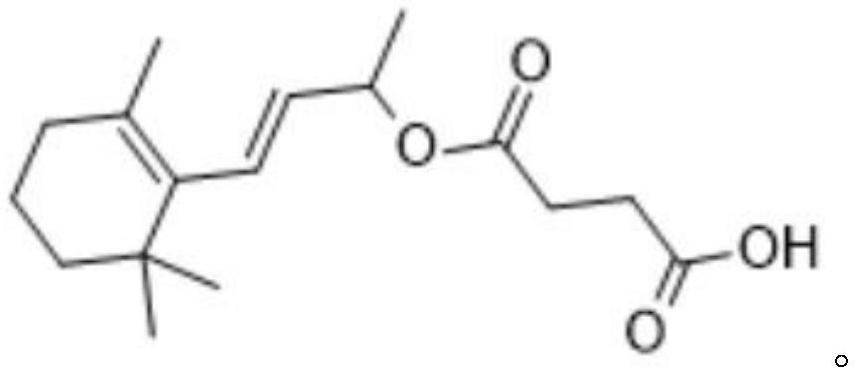
[0102] This embodiment provides a kind of beta-ionone hapten, and its structural formula is as follows:
[0103]
[0104] It can be seen that the hapten provided by the present invention directly introduces hydroxyl and carboxyl groups through the reduction of the aldehyde group on the β-ionone, retains the chemical structure and characteristics of the β-ionone to the greatest extent, and can be directly combined with the carrier. The protein coupling laid the foundation for the subsequent preparation of high-specificity and high-sensitivity β-ionone antibodies.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com