Preparation method of prothioconazole intermediate 3, 5-dichloro-2-pentanone
A technology for prothioconazole and intermediates, which is applied in the field of preparation of prothioconazole intermediate 3,5-dichloro-2-pentanone, which can solve complex process, short process flow, large amount of waste acid and water, etc. problem, to achieve the effect of simple process, avoiding separation process and reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
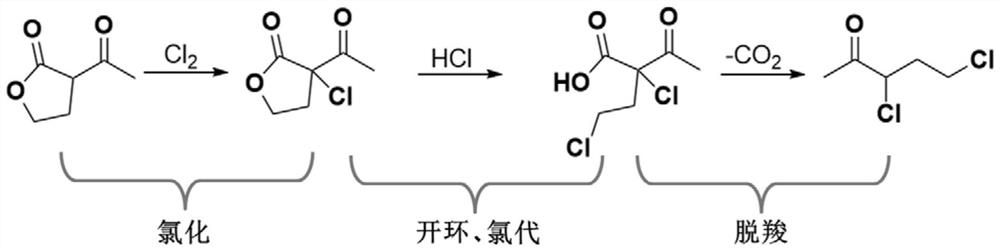
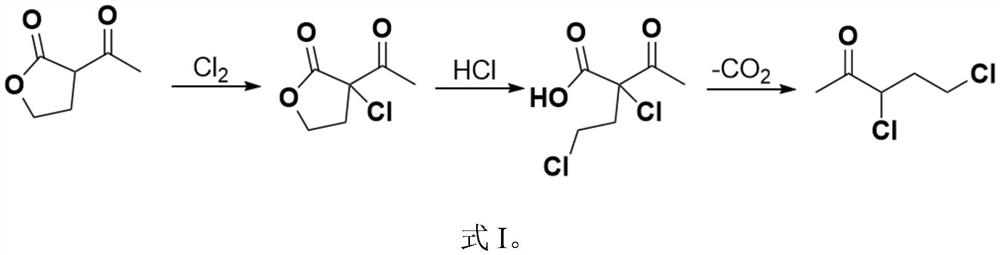
[0071] The present embodiment provides a preparation method of 3,5-dichloro-2-pentanone, which specifically includes the following steps:
[0072] (1) Synthesis of α-chloro-α-acetyl-γ-butyrolactone
[0073] Add 128.8g α-acetyl-γ-butyrolactone (1.0mol, 99.5%, 1.0eq) to a 500mL four-necked reaction flask equipped with a mechanical stirring, a thermometer and a tail gas trapping device, and cool to 0°C;
[0074] Begin to pass 74.5g chlorine gas (1.05mol, 99.9%, 1.05eq), control the temperature not to exceed 5 ℃, and use 3h to pass through; after the chlorine pass is completed, continue to keep the temperature for 1h to complete the reaction;
[0075] 178.4g of reaction solution (containing about 13.4g of HCl, 1.0mol of α-chloro-α-acetyl-γ-butyrolactone) was obtained by weighing, and the reaction was directly entered into the next step without transferring materials;
[0076] The tail gas trapping device collected 24.9g (0.68mol, 99.0%) of HCl gas, which can be directly used in t...
Embodiment 2
[0086] The present embodiment provides a preparation method of 3,5-dichloro-2-pentanone, which specifically includes the following steps:
[0087] (1) Synthesis of α-chloro-α-acetyl-γ-butyrolactone
[0088]Add 128.8g α-acetyl-γ-butyrolactone (1.0mol, 99.5%, 1.0eq) to a 500mL four-necked reaction flask equipped with a mechanical stirring, a thermometer and a tail gas trapping device, and cool to 0°C;
[0089] Begin to pass 78.1g chlorine gas (1.1mol, 99.9%, 1.05eq), control the temperature not to exceed 5 ℃, and use 4h to pass through; after the end of chlorine pass, continue to keep warm for 1h and the reaction ends;
[0090] 180.0g of reaction solution (containing about 13.4g of HCl, 1.0mol of α-chloro-α-acetyl-γ-butyrolactone) was obtained by weighing, and the reaction was directly entered into the next step without transferring materials;
[0091] The tail gas was collected to obtain 26.9 g (0.73 mol, 99.0%) of HCl gas, which could be directly used in the next reaction;
...
Embodiment 3
[0098] The present embodiment provides a preparation method of 3,5-dichloro-2-pentanone, which specifically includes the following steps:
[0099] (1) Synthesis of α-chloro-α-acetyl-γ-butyrolactone
[0100] Add 128.8g α-acetyl-γ-butyrolactone (1.0mol, 99.5%, 1.0eq) to a 500mL four-necked reaction flask equipped with a mechanical stirring, a thermometer and a tail gas trapping device, and cool to 0°C;
[0101] Begin to pass 74.5g chlorine gas (1.05mol, 99.9%, 1.05eq), control the temperature not to exceed 5 ℃, and use 4h to pass through; after the chlorine pass ends, continue to keep warm for 1h and the reaction ends;
[0102] 178.5g of reaction solution (containing about 13.4g of HCl, 1.0mol of α-chloro-α-acetyl-γ-butyrolactone) were obtained by weighing, and the reaction was directly entered into the next step without transferring materials;
[0103] The tail gas was collected to obtain HCl gas 24.9g (0.68mol, 99.0%);
[0104] (2) Synthesis of 3,5-dichloro-2-pentanone
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com